Eight Dots Around An Elemental Symbol Represent What

Molecule Definition Examples Structures Facts Britannica
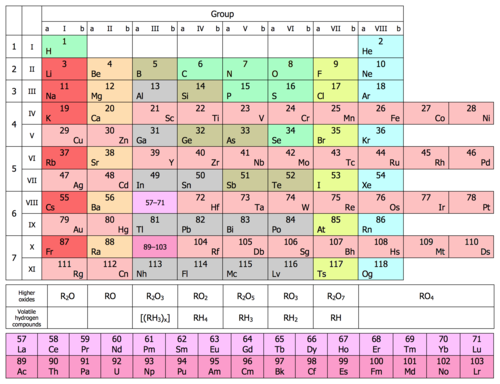
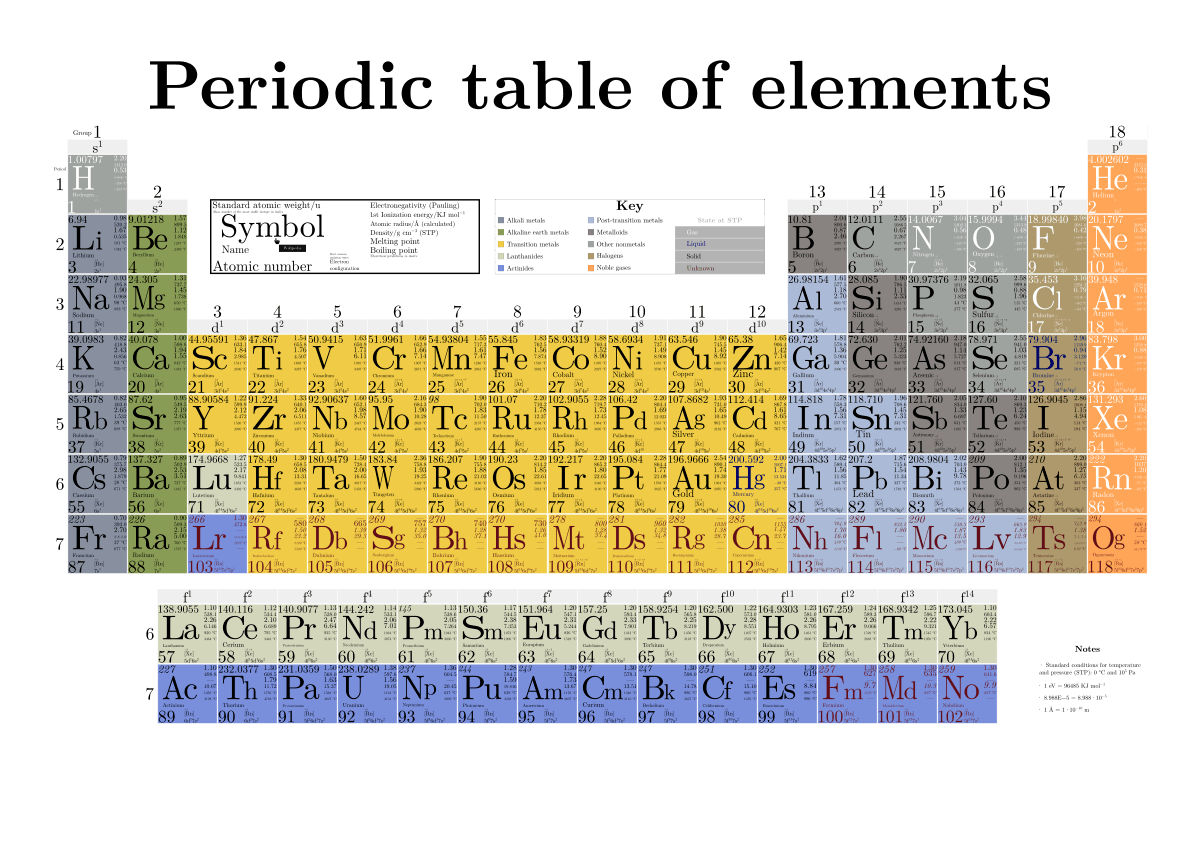
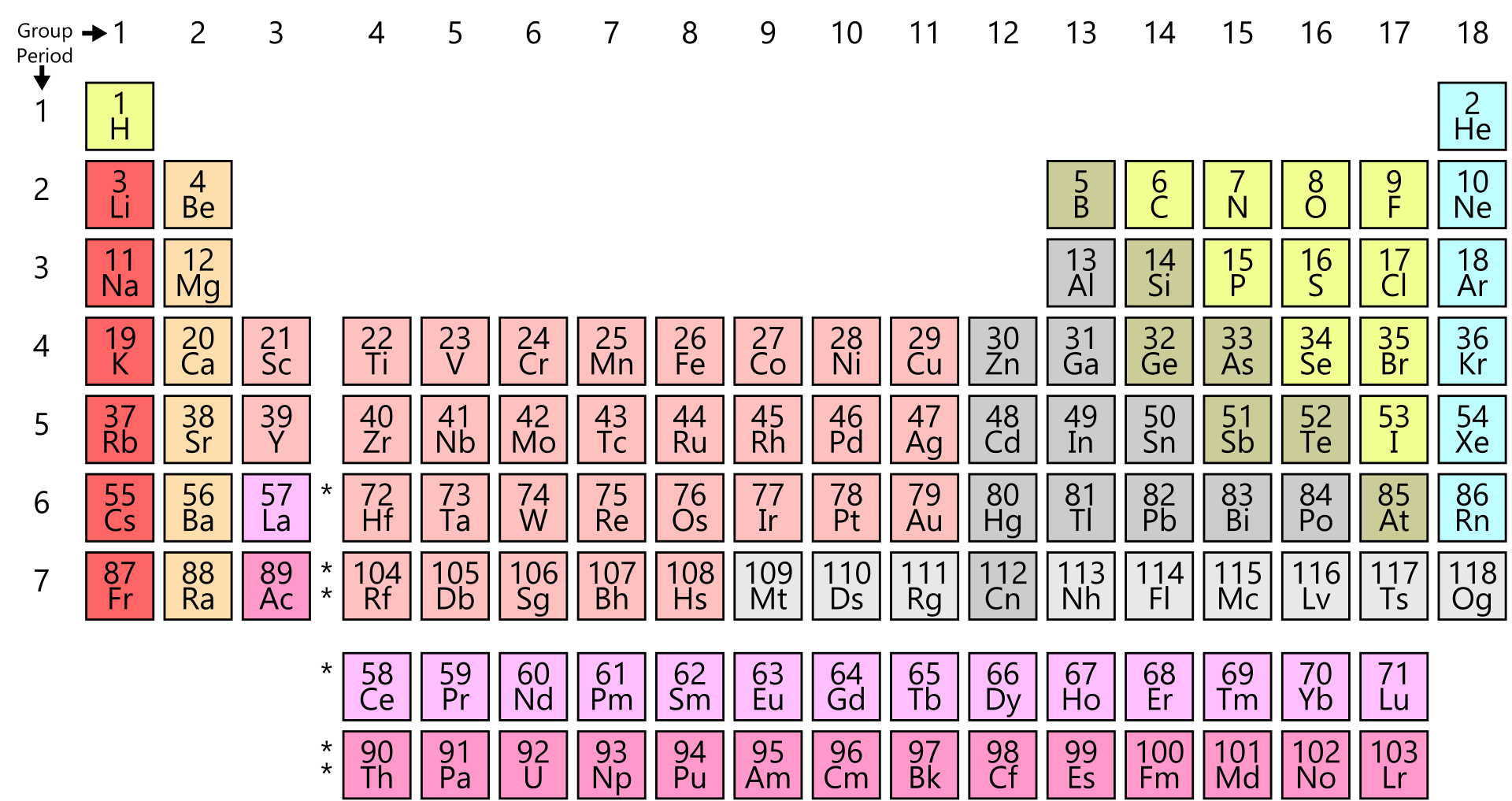
Periodic Table Wikipedia
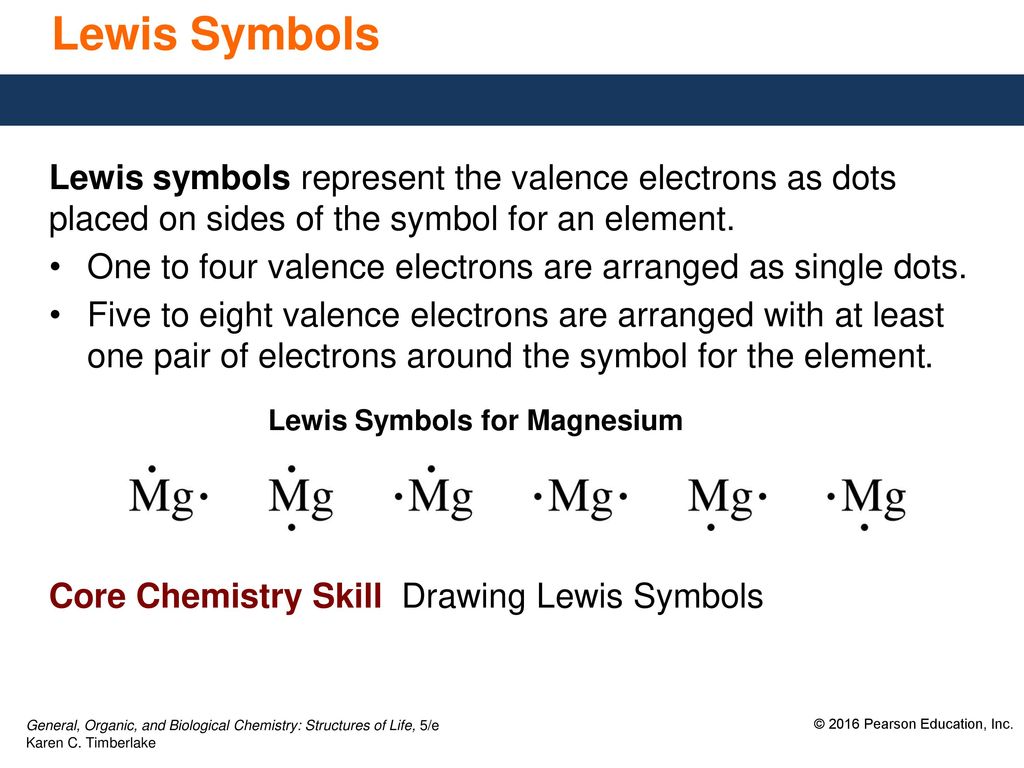
4 8 Trends In Periodic Properties Ppt Download

6 1 Lewis Electron Dot Diagrams Introductory Chemistry
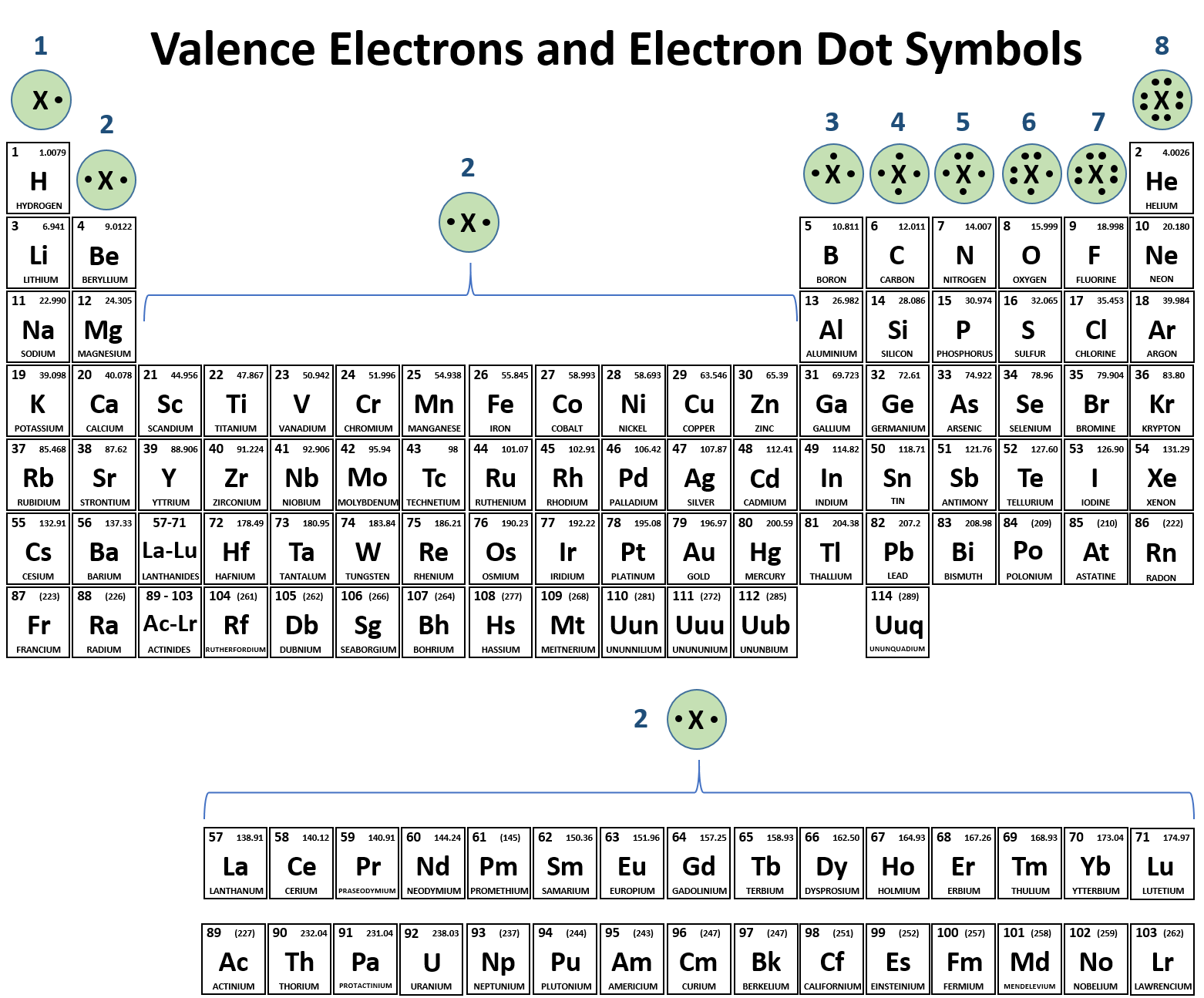
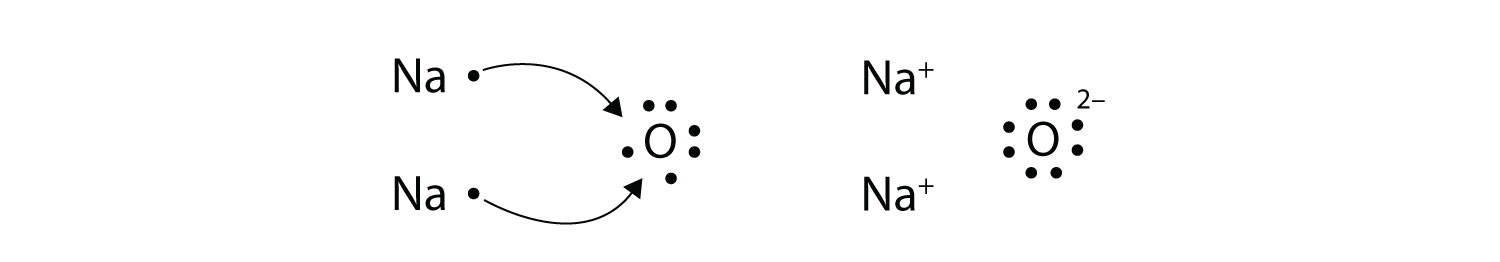
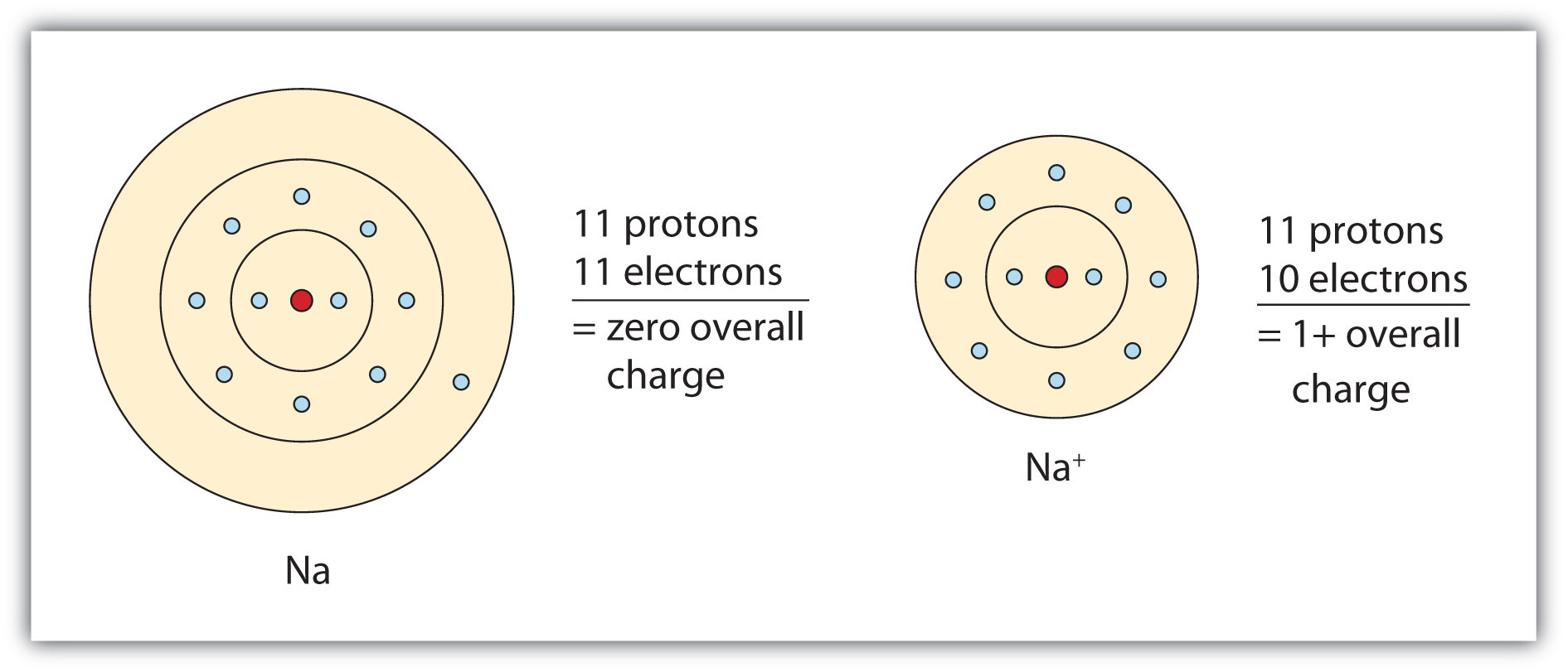
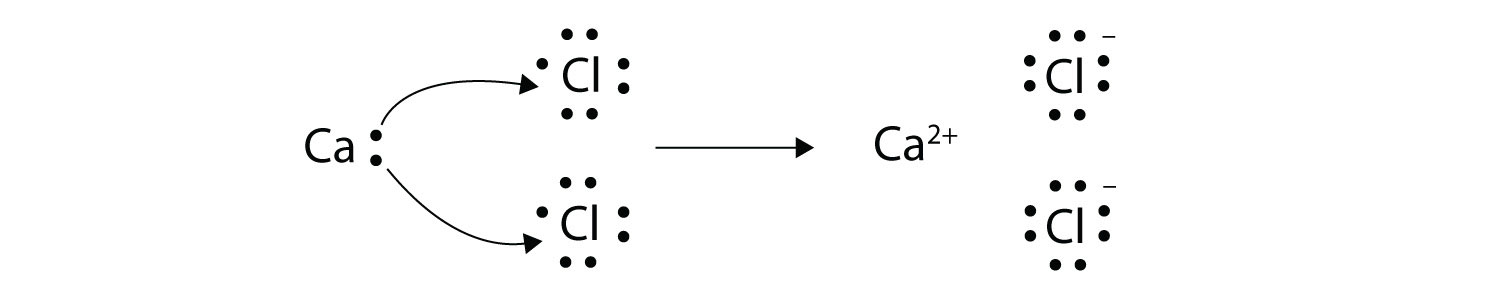
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry

Lewis Dot Symbols And Lewis Structures Boundless Chemistry
The number eight frequently represents beginnings, resurrection, salvation , and superabundance This has to do, in part, with the fact that the number seven is a number of completion The eighth day, for example, is the first day of a new sevenday week, and a Jewish child enters into God's Covenant on the eighth day of life via circumcision.

Eight dots around an elemental symbol represent what. Each of the four sides around the elemental symbol represents one of the orbitals in the outer shell Here are the rules Rule #1 No side can have more than two dots because each orbital can only hold two electrons Rule #2 When filling the sides of the element symbol each side gets one dot before doubling upthat's Hund's Rule, not ours. As an example, an oxygen atom has six electrons in its outer shell In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons The two pairs would be opposite each other around the O symbol and the two single electrons would be on the other sides of the atom, opposite each other. (Helium is an exception, with both valence electrons paired up on the same side of the symbol) 4 For assigning more than 4 valence electrons, start pairing up electrons on the four sides to a maximum of eight electrons The number of dots you have around the symbol represents the actual number of valence electrons for the element.
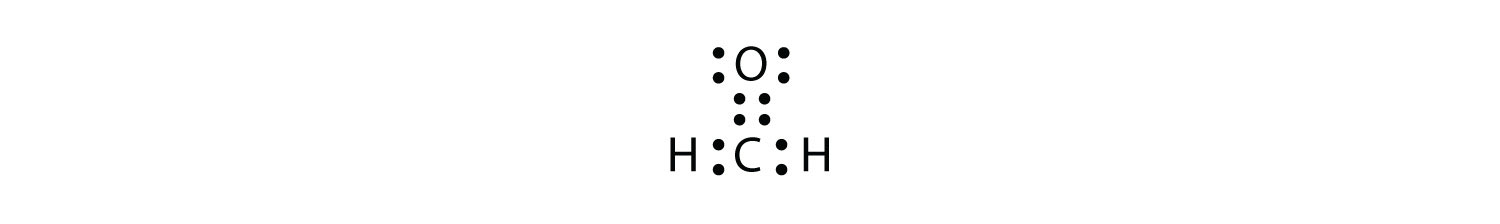
(Helium is an exception, with both valence electrons paired up on the same side of the symbol) 4 For assigning more than 4 valence electrons, start pairing up electrons on the four sides to a maximum of eight electrons The number of dots you have around the symbol represents the actual number of valence electrons for the element. The Lewis dot notation consists of the kernel (the nucleus and all of its inner electrons that is represented by the elemental symbol) and the valence electrons represented by dots Check out the lewis dot structure of carbon below. Octet Rule Atoms tend to gain, lose, or share electrons until they are surrounded by eight electrons (4 electron pairs) Using Lewis dot structures and the octet rule, we can predict and represent the electronic structure of covalently bonded molecules.
For instance, it appears in the cross of Saint Boniface, wrapped around the bars of a Latin cross However, John Wallis is credited with introducing the infinity symbol with its mathematical meaning in 1655, in his De sectionibus conicis Wallis did not explain his choice of this symbol, but it has been conjectured to be a variant form. Some believe that a circle placed around a pentagram symbolizes the connection between the five elements Air, fire, earth, water, and spirit The symbol is most used by Wiccans, also known as practitioners of modern witchcraft. The Greeks proposed the existence of five basic elements Of these, four were the physical elements—fire, air, water, and earth—of which the entire world is composed Alchemists eventually associated four triangular symbols to represent these elements.
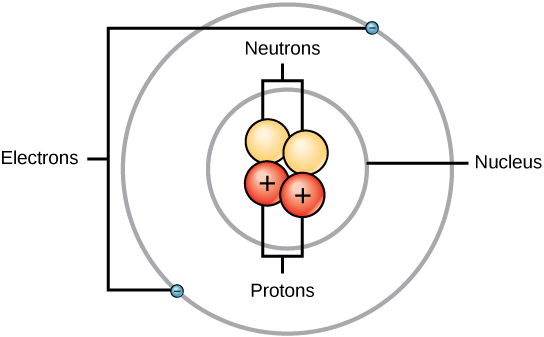
In the Lewis symbol for an atom, the chemical symbol of the element (as found on the periodic table) is written, and the valence electrons are represented as dots surrounding it Only the electrons in the valence level are shown using this notation. In a Lews dot structure, the elemental symbol is used to represent the molecule's nucleus The electrons are then represented by the dots Xenon has 8 dots (4 pairs of dots) around the letters Xe. (See sun symbol below the picture of the Eye of Horus) A dot or point in the center of a circle symbolizes the blending of male and female forces (See air, which also represents spirit, among the symbols for Elements) Hindus call the midpoint in a circle the bindu the spark of (masculine) life within the cosmic womb.
Octet Rule Atoms tend to gain, lose, or share electrons until they are surrounded by eight electrons (4 electron pairs) Using Lewis dot structures and the octet rule, we can predict and represent the electronic structure of covalently bonded molecules. How many electrons should Oxygen have around its Lewis dot model?. •As a pictorial understanding of where the electrons are in an atom, we represent the electrons as dots around the symbol for the element •The number of valence electrons available for bonding are indicated by unpaired dots •These symbols are called Lewis symbols, or Lewis electrondot symbols.
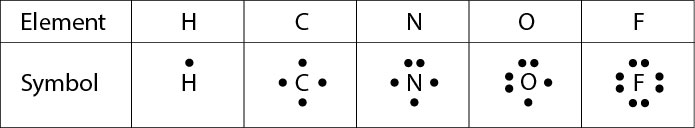
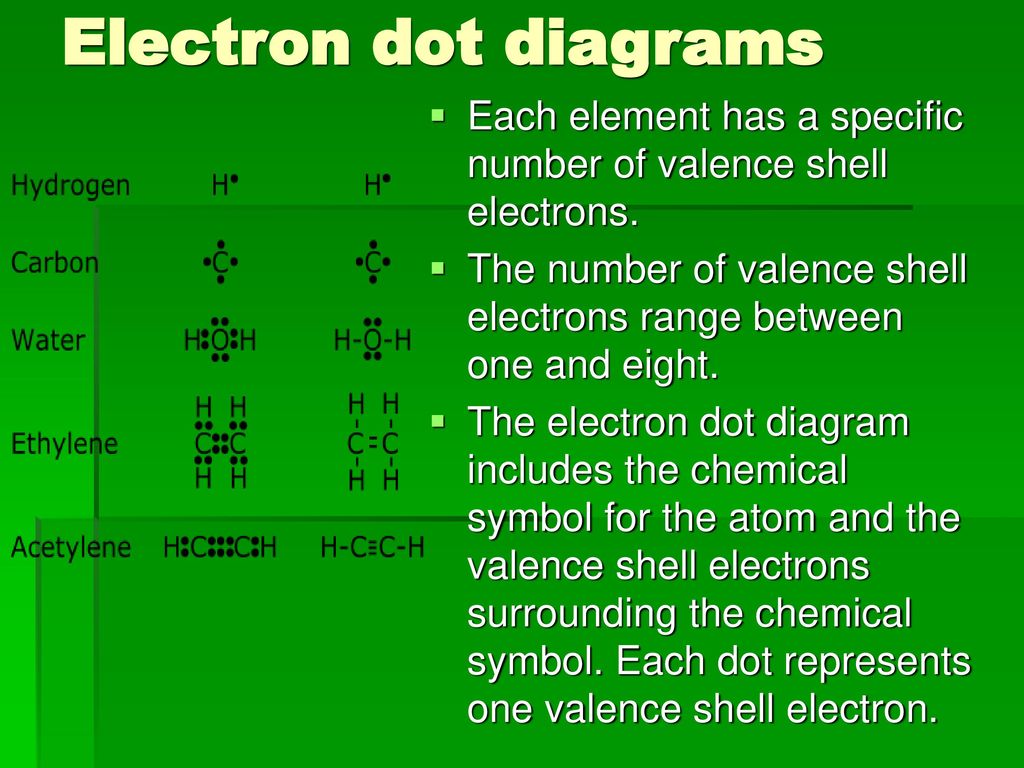
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element The number of dots equals the number of valence electrons in the atom. Also called a Lewis valence electron dot structure any atom with more or less electrons than its neutral state. He represents these valence electrons as "dots" around the four sides of the elemental symbol Depending on how your teacher was taught, this may be slightly different The first 2 valence electron go together (I was taught to place them on top), then one on each side going clockwise (3 o'clock, 6 o'clock then 9 o'clock).
Question ElectronDot Formulas For Elements An Erector Dot Formula, Or Lewis Electrondot Structure, Can Be Used To Represent The Number Of Valence Electrons In The Atom Of An Element The Symbol Of An Element Is Used To Represent The Core Of The Atom, And The Valence Electrons Are Denoted Using Dots Arranged Around The Symbol The Maximum Number Of Electron Dots. Each of the four sides around the elemental symbol represents one of the orbitals in the outer shell Here are the rules Rule #1 No side can have more than two dots because each orbital can only hold two electrons Rule #2 When filling the sides of the element symbol each side gets one dot before doubling upthat's Hund's Rule, not ours. A The element symbol represents the nucleus and filled energy levels of the atom b Dots represent the valence electrons of the atom c Four valence orbitals are represented by the four sides of.
We represent an electron dot structure by using the element symbol Start from the top of the element symbol, then add dots in a clockwise manner to complete the number of valence electrons The maximum number of electrons can be eight It defines the nature of the bond and position of atoms of the molecule which are connected in the molecule. As a crucial element in the development of civilization in the history of mankind, fire is widely seen as a symbol with multiple meanings a flame can signify wisdom and knowledge, while a raging fire is often used to symbolize fear, pain, anger, punishment, destruction and even death Such associations likely hearken back to earliest days of. The symbol represents the inner electrons and atomic nucleus;.
These emanations represent eight forms of wealth monetary, ability to transport, endless prosperity, victory, patience, health and nourishment, knowledge, and family Overlapping Squares Octagrams formed from overlapping squares often emphasize duality yin and yang, male and female, spiritual and material. In Babylonian symbolism, the goddess Ishtar is represented by an eightpointed starburst, and she is associated with the planet of Venus Today, some people equate the Greek Aphrodite, whom the Romans equated with their Venus, with Ishtar Both goddesses represent lust and sexuality, although Ishtar also represents fertility and war. A notation that depicts valence electrons as dots around the atomic symbol of the element;.
The eight valence electrons, a full outer s and p sublevel, give the noble gases their special stability When examining chemical bonding, it is necessary to keep track of the valence electrons of each atom Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol A beryllium atom, with two valence electrons, would have the electron dot diagram below. A electron dot diagram is defined as the diagram in which dots are placed around the symbol of an element These dots represent the number of valence electrons present in an element For example, atomic number of chlorine is 17 and it has 7 valence electrons. Answer Electron dot structure valence electrons are represented by dots placed around the chemical symbol Electrons are placed up to two on each side of the elemental symbol for a maximum of eight, which is the number of electrons in a filled s and p shell.
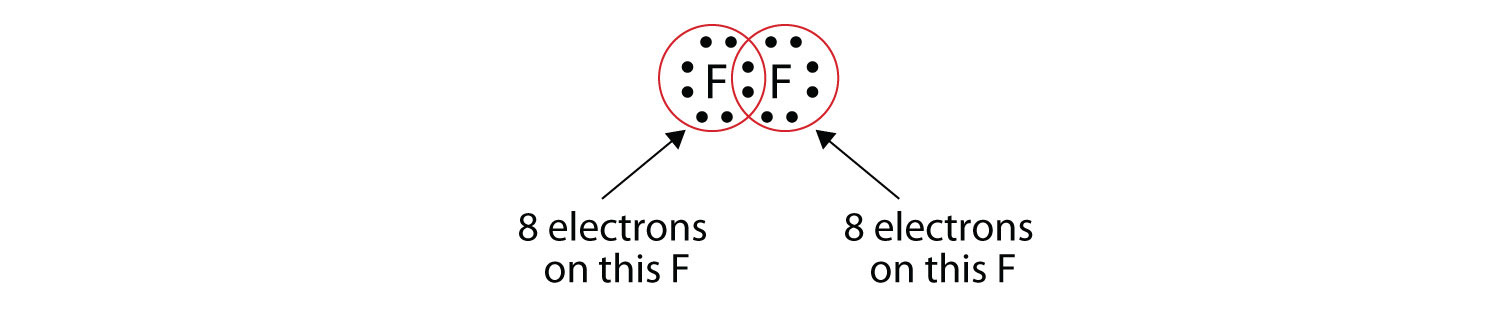
The four dots represent the four concepts of Karma Right thought, right words, right actions, and right understanding Swastika Symbol Meaning – Greece The above shown symbol is an artistic rendition of a tetraskelion, which is the Greek version of the swastika. First, they show only valence electrons Second, instead of having a circle around the chemical symbol to represent the electron shell, they have up to eight dots around the symbol;. As a crucial element in the development of civilization in the history of mankind, fire is widely seen as a symbol with multiple meanings a flame can signify wisdom and knowledge, while a raging fire is often used to symbolize fear, pain, anger, punishment, destruction and even death Such associations likely hearken back to earliest days of.
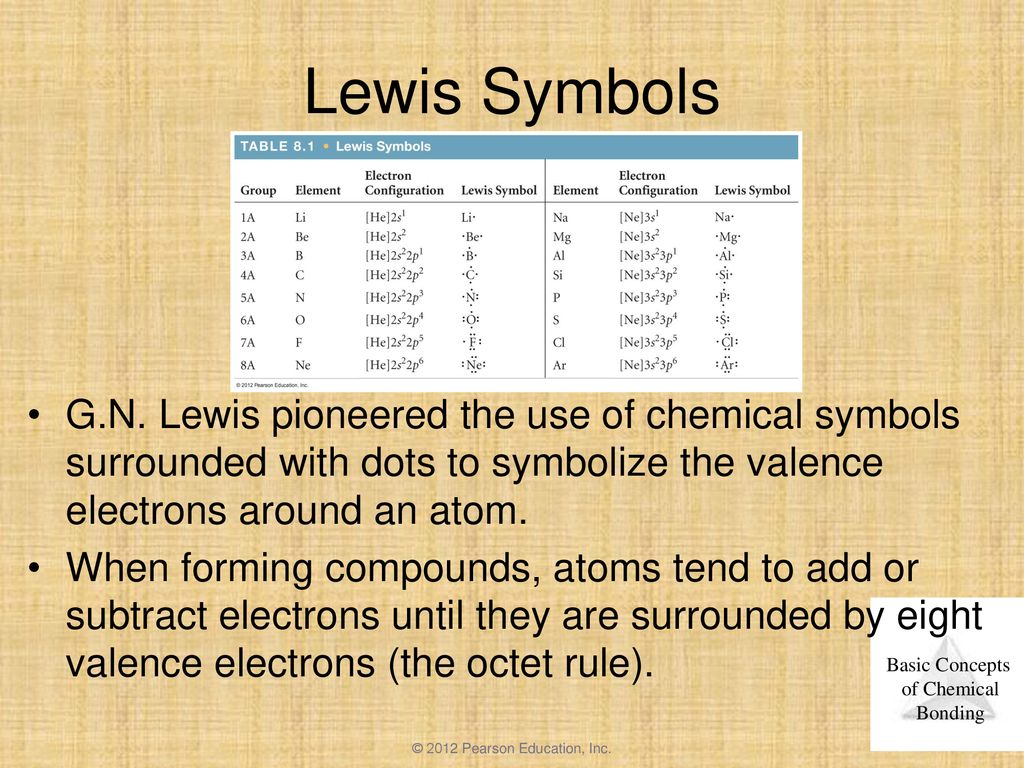
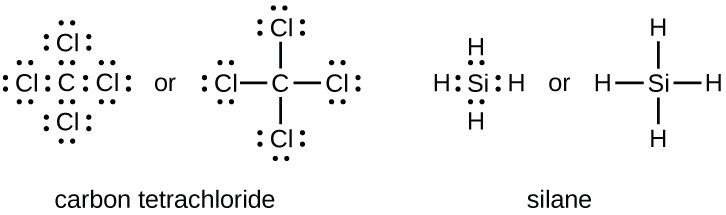
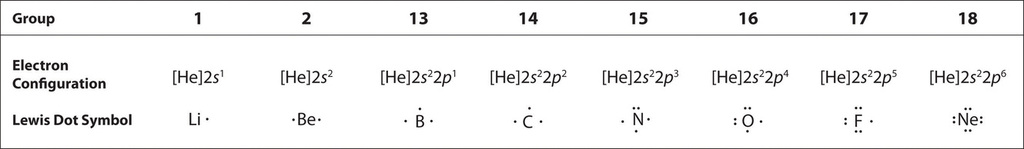
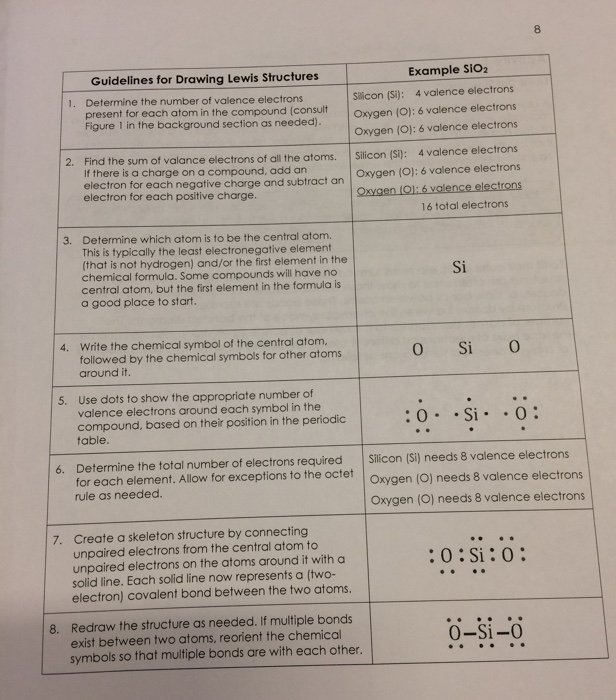
The element with the least number of atoms is usually the central element Draw a tentative molecular and electron arrangement attaching other atoms with single bonds as the first guess Single bonds represented with a line represent 2 electrons 3 Add electrons as dots to get octets around atoms. For instance, it appears in the cross of Saint Boniface, wrapped around the bars of a Latin cross However, John Wallis is credited with introducing the infinity symbol with its mathematical meaning in 1655, in his De sectionibus conicis Wallis did not explain his choice of this symbol, but it has been conjectured to be a variant form. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table Figure 1 Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom.
The shape of a sideways figure eight has a long pedigree;. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table Figure 1 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Electron dot structurevalence electrons are represented by dots around the symbol of chemistry.
Lewis symbols are diagrams showing the number of valence electrons of a specific element with dots indicating lone pairs How do electron dot structures represent shared electrons?. The symbol of an element is used to represent the core of the atom, and the valence electrons are denoted using dots arranged around the symbol The maximum number of electron dots that an element can have is eight The electrons are added one at a time moving around the core atom until each side of the atom has a single dot before the electrons are paired The first four electrons are arranged singly. Some believe that a circle placed around a pentagram symbolizes the connection between the five elements Air, fire, earth, water, and spirit The symbol is most used by Wiccans, also known as practitioners of modern witchcraft.
To write an element’s Lewis dot symbol, we place dots representing its valence electrons, one at a time, around the element’s chemical symbol Up to four dots are placed above, below, to the left, and to the right of the symbol (in any order, as long as elements with four or fewer valence electrons have no more than one dot in each position). A electron dot diagram is defined as the diagram in which dots are placed around the symbol of an element These dots represent the number of valence electrons present in an element For example, atomic number of chlorine is 17 and it has 7 valence electrons. Typically, the dots of the eightdot braille cell are numbered 1, 2, 3, 7 downward on the left and 4, 5, 6, 8 downward on the right BANA recognizes that eightdot braille systems have proven to be extremely useful, particularly in the technical areas such as mathematics and the sciences.
The symbol "Ar" with 8 dots around it. Each dot represents a valence electron These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. 8 dots unless it needs less 8 dots always s Question 29 SURVEY 30 seconds Q The inner (kernel) electrons in a Lewis Diagram are represented by answer choices dots brackets charge element symbol s Question 30 SURVEY 30 seconds Q Ions always have.
Lewis Dot Diagrams • AKA Electron Dot Diagrams • Consists of – Element symbol in the center – 18 dots around the symbol representing valence electrons Lewis Dot Diagrams • Steps 1 Write the element’s symbol 2. A full octet of electrons (8 dots) is a stable configuration Oxygen would need 2 more electrons to be stable Well that is what it does Takes 2 electrons and becomes stable It now has a 2 charge Metal ions are a little different They get to 8 electrons by losing their valence electrons and using the full inner electron level. For assigning more than 4 valence electrons, start pairing up electrons on the four sides to a maximum of eight electrons The number of dots you have around the symbol represents the actual number of valence electrons for the element The end result is, for representative elements belonging to BACK TO MAIN PAGE.
The only dots that surround an element ARE electrons Sometimes, when scientists are trying to write about an element, they will draw a number of dots around the element's symbol to show how many. Count the dots to make sure that all of the valence electrons are represented Draw dots around the chemical symbol to represent the valence electrons of the atom Use the periodic table to find the chemical symbol of the atom and the number of electrons in the valence shell Write the chemical symbol of the atom.
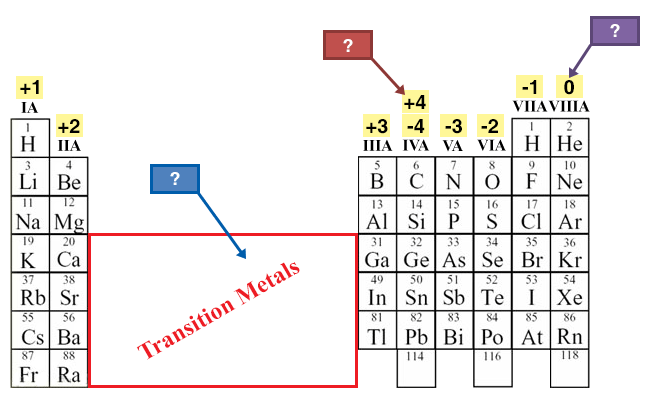
Ionic Bonds Electron Dot Formulas Texas Gateway
Covalent Compounds Manoa Hawaii Edu Exploringourfluidearth
Q Tbn And9gctx2unihrwvsl45ij5h Bp2grxrgaokdparonvakfmrfmh9cyz4 Usqp Cau
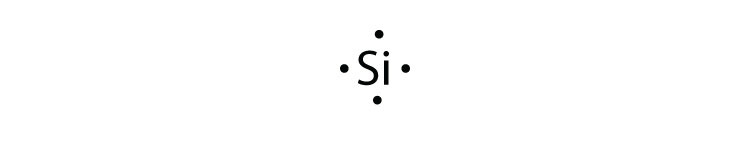
Lewis Electron Dot Diagrams

Solved Chapter 10 Problem Sets True Or False 1 Bonding Chegg Com
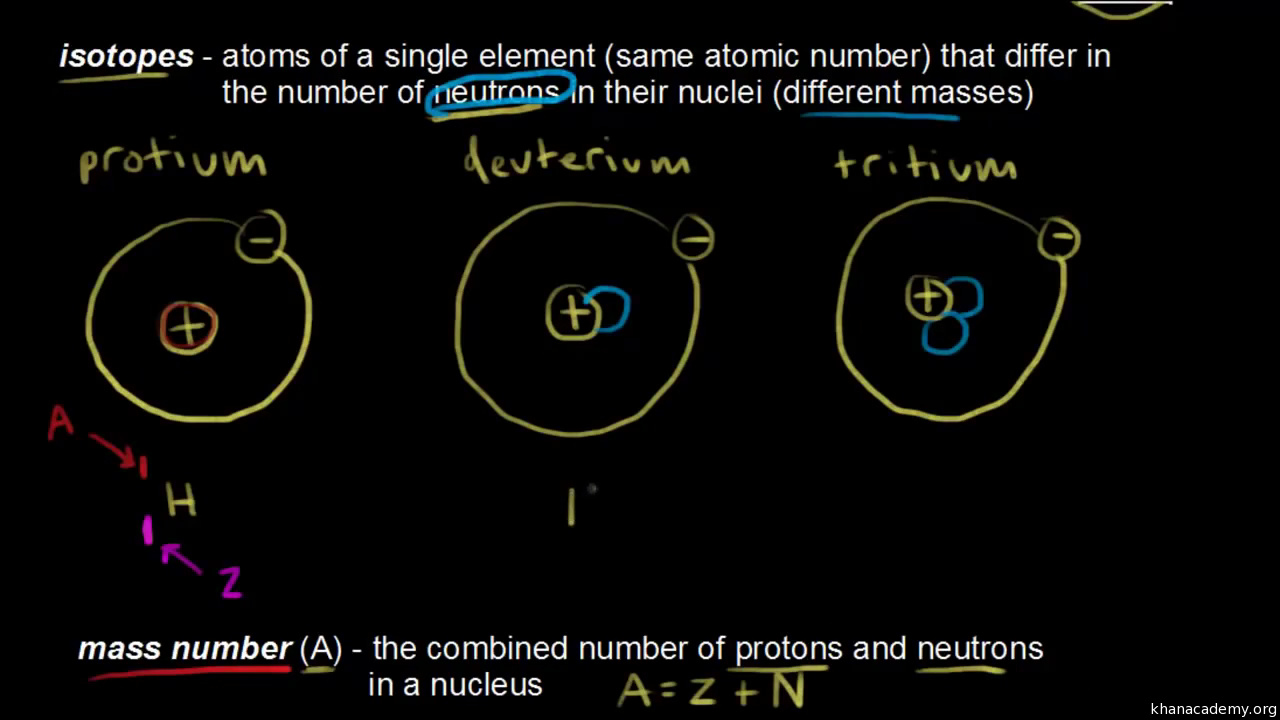
Atomic Number Mass Number Definition Videos Calculations With Examples And Faqs

Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library
2

Diatomic Molecule Wikipedia
/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)
Lewis Structure Definition And Example
Chemical Bonds
Chapter 8 Basic Concepts Of Chemical Bonding Ppt Download

Chemical Bonds

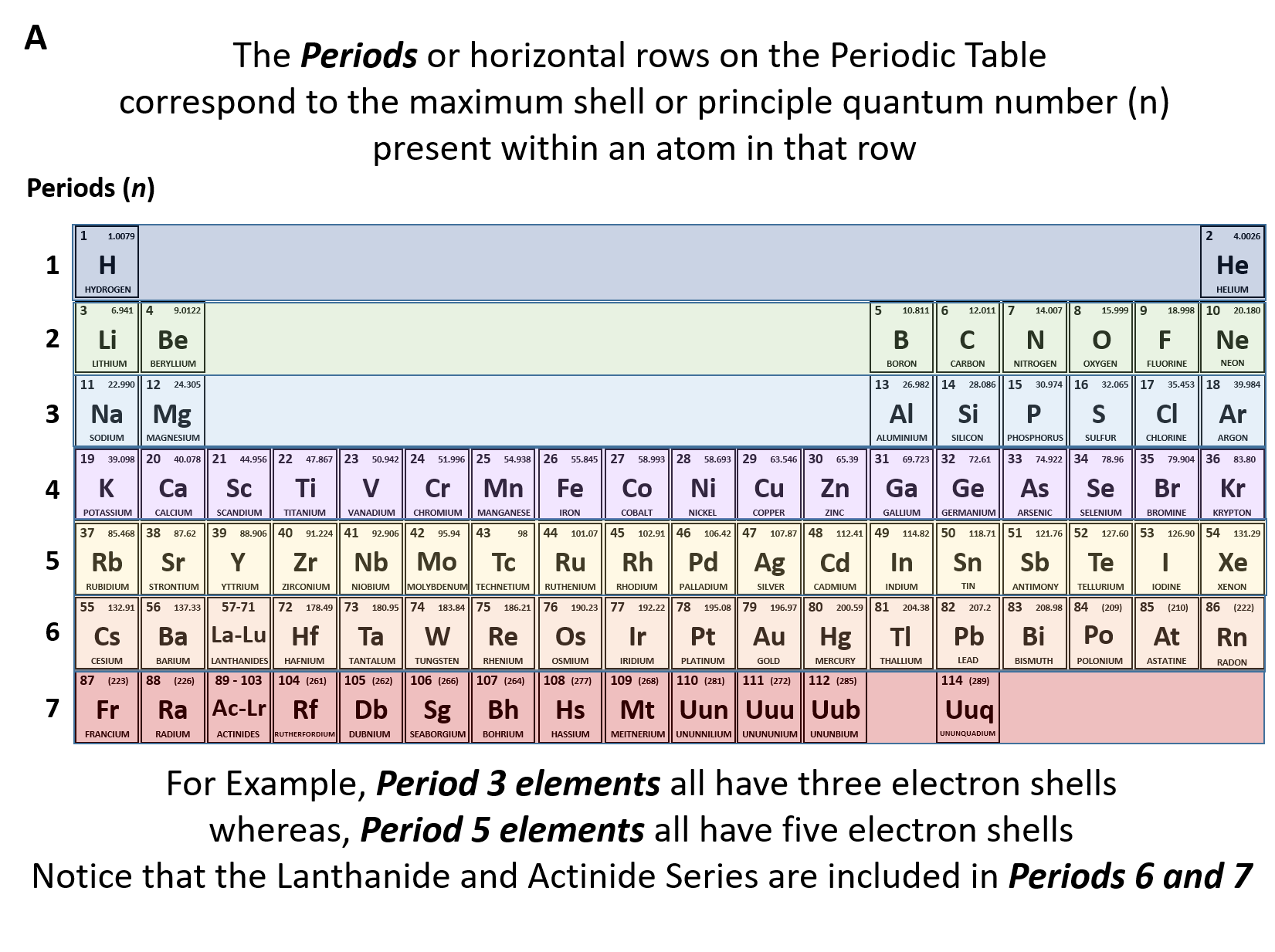
The Periodic Table In A Previous Section The Periodic Table Was Introduced As A List Of The Elements We Also Pointed Out That The Design Of The Periodic Table Separates The Metals From The Nonmetals In This Section We Will Show How The Various Features Of The
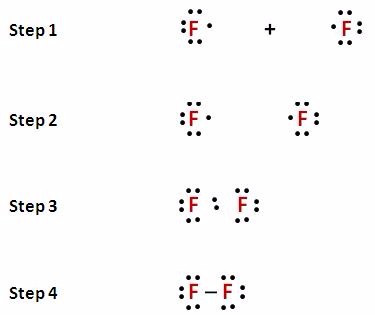
Lewis Dot Symbols And Lewis Structures Boundless Chemistry
8 3 Lewis Symbols And Structures Chemistry Libretexts
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

6 1 Lewis Electron Dot Diagrams Introductory Chemistry
Average Atomic Mass Video Khan Academy

Chemistry I Atoms And Molecules
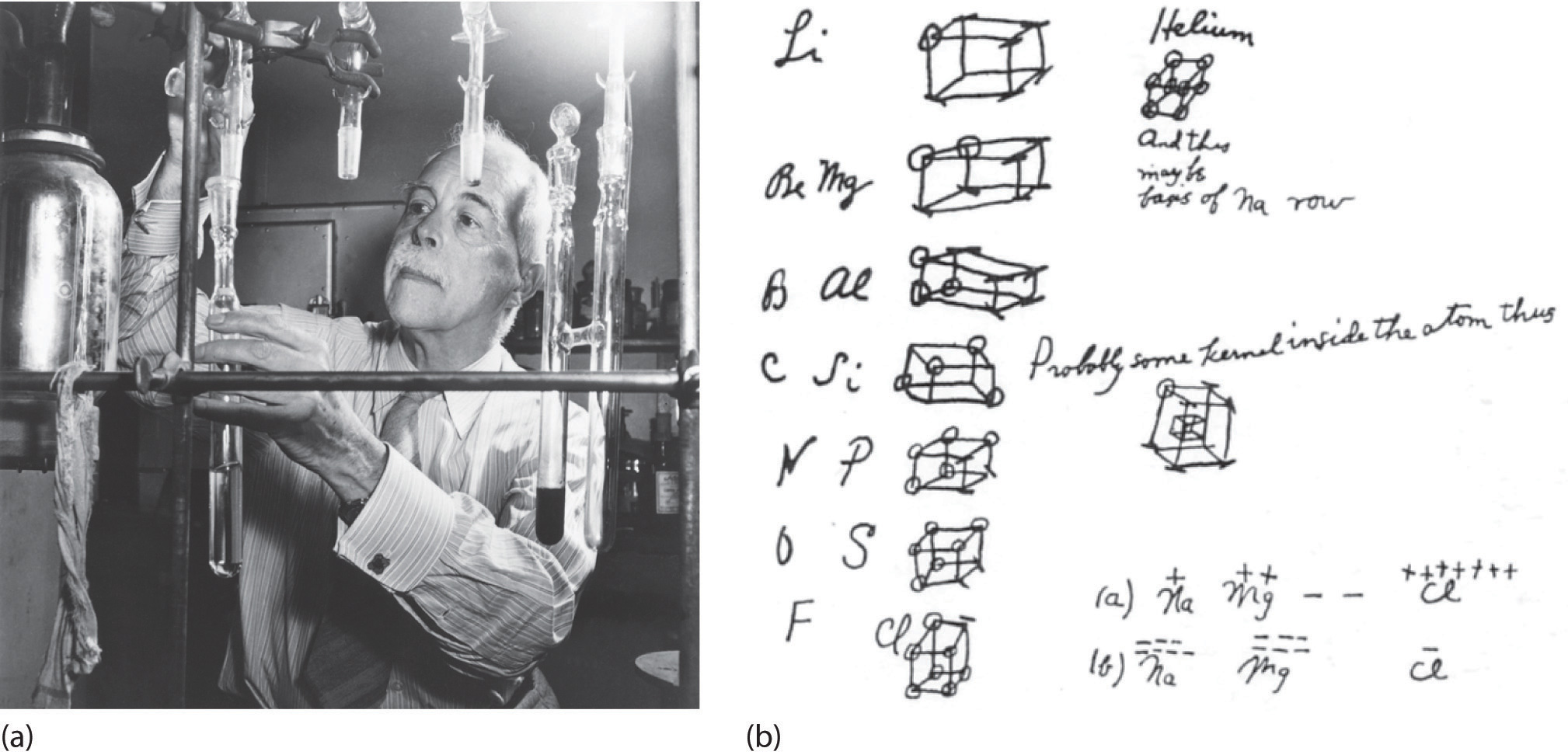
Lewis Electron Dot Symbols
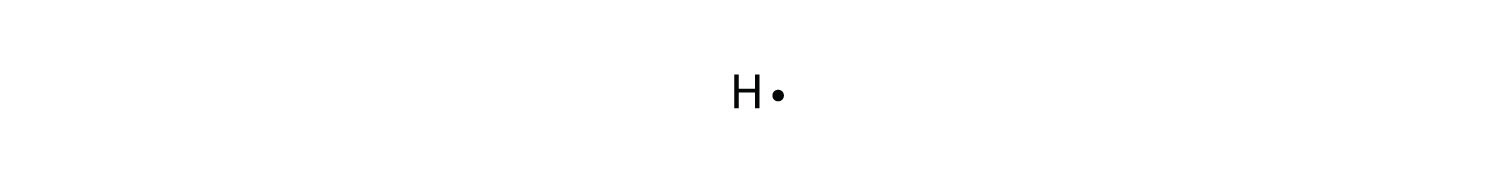
Lewis Electron Dot Diagrams

Lewis Symbols And Structures Introductory Chemistry
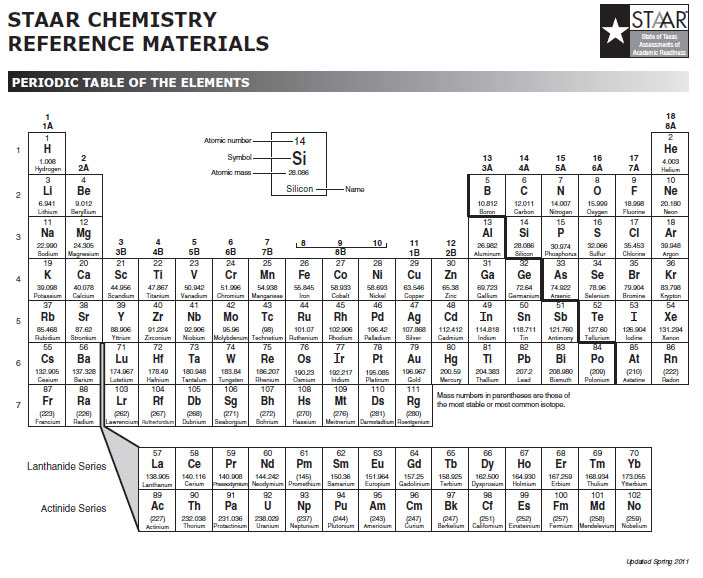
Ionic Bonds Electron Dot Formulas Texas Gateway

Lewis Symbols And Structures Introductory Chemistry
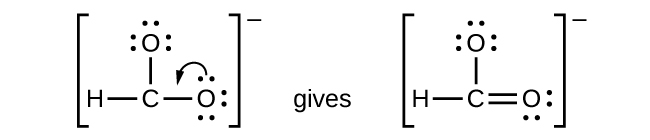
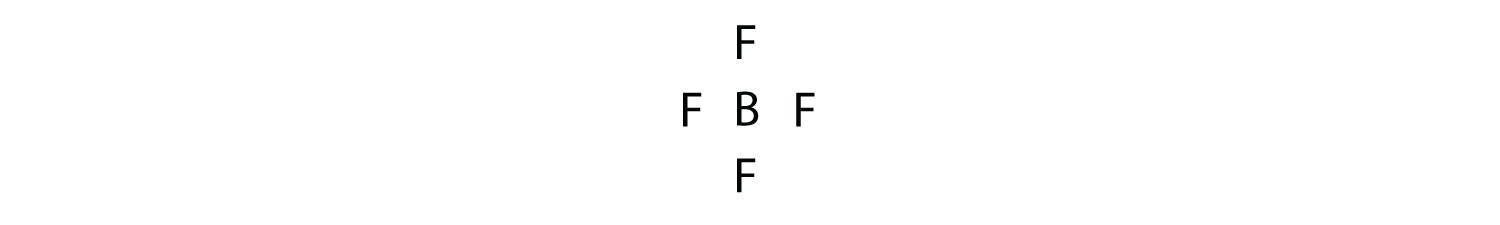
Lewis Symbols And Structures Chemistry 2e

Chemistry I Atoms And Molecules
Chemical Bonds

Electron Dot Diagrams Chemistry For Non Majors

Glava 9 Br Section A Br Lewis Electron Dot Diagrams
Lewis Dot Structure Chemistry Ppt Download
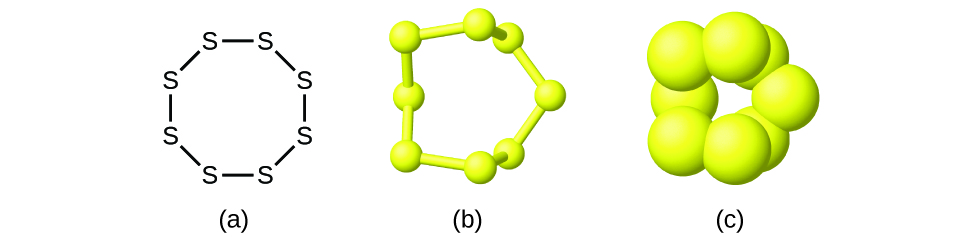
2 4 Chemical Formulas Chemistry

Lewis Dot Symbols And Lewis Structures Boundless Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
Images Topperlearning Com Topper Revisionnotes 4253 Topper 21 101 3 2 30 58 Chemical Bonding Up 3373 Pdf V 0 0 1
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How To Draw A Lewis Structure

7 3 Lewis Symbols And Structures Chemistry
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library

Molecule Definition Examples Structures Facts Britannica
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library

Lewis Electron Dot Symbols
Solved 2 The Atomic Number Of Fluorine Is 9 How Many El Chegg Com

Chapter 6 P Bonding Ppt Download
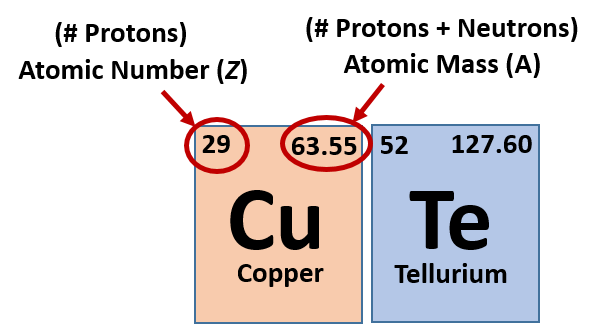
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

7 3 Lewis Symbols And Structures Chemistry

Lewis Symbols And Structures Introductory Chemistry

Notation Orbital And Lewis Dot Help Types Of Bonds And Orbitals Study Guide Shmoop
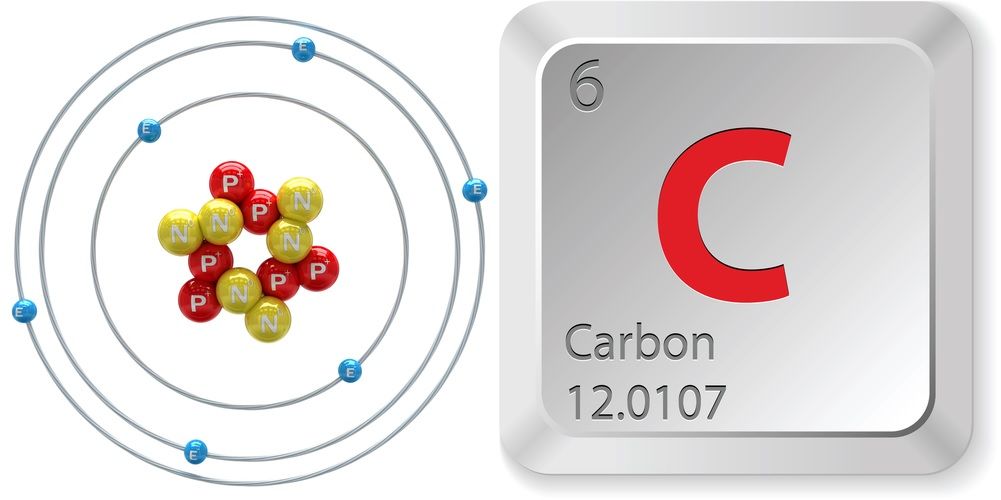
Carbon Element Facts Discovery Atomic Structure Uses Live Science
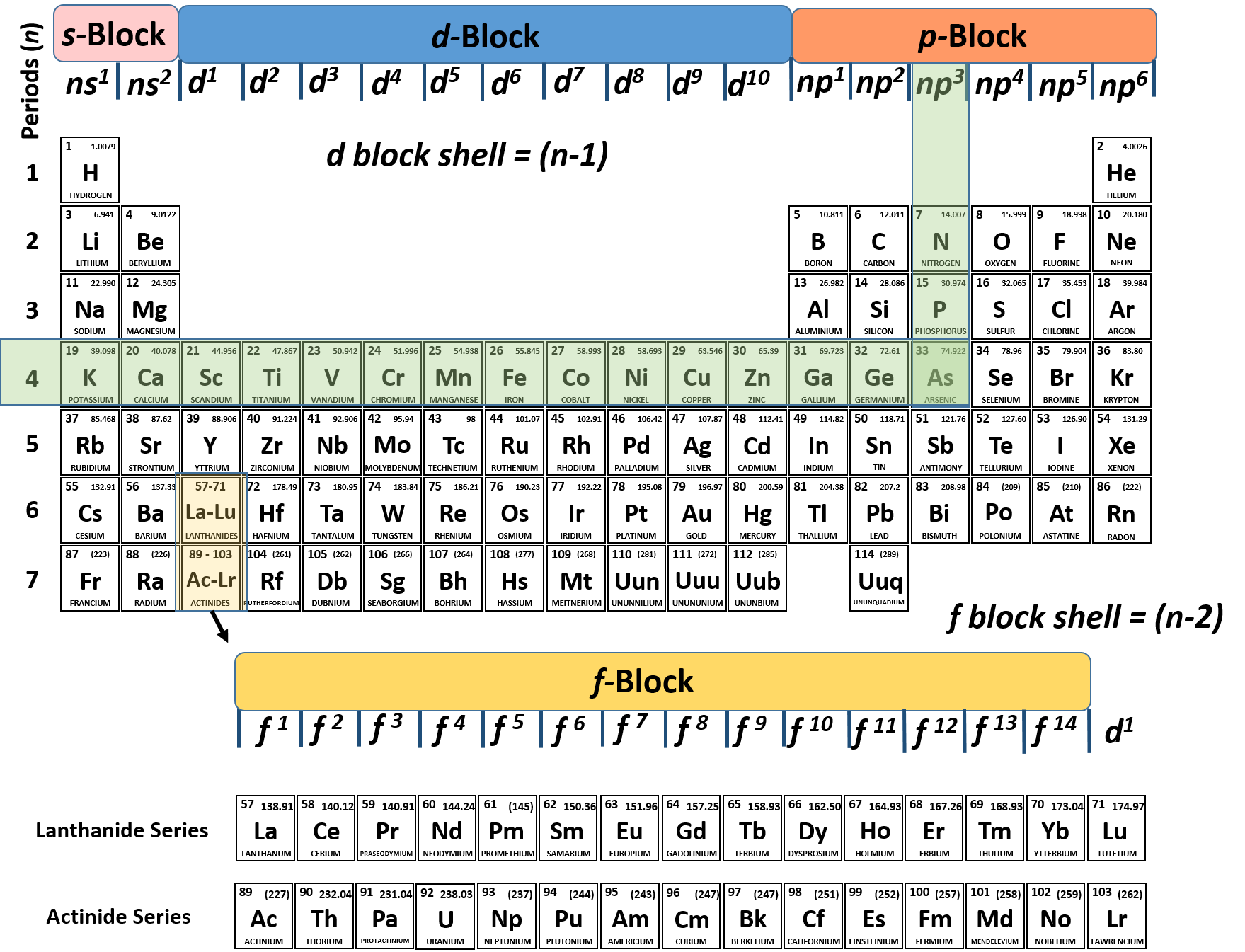
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
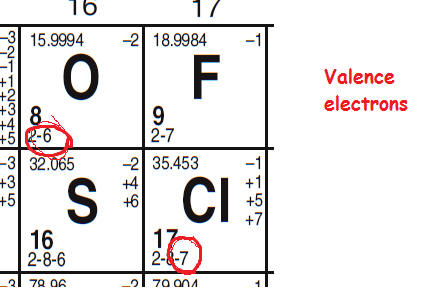
Valence Electrons And Lewis Electron Dot Of Atoms And Ions
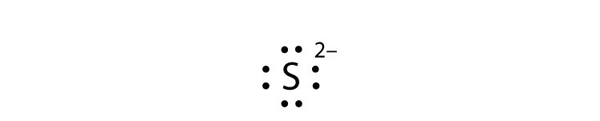
Lewis Electron Dot Diagrams Introductory Chemistry 1st Canadian Edition

Lewis Structures Learn How To Draw Lewis Structures Albert Io

Drawing Dot Structures Video Khan Academy
Powerschool Learning 8th Grade Science Sec 3 Atomic Number
Ions
Atoms Bonding And The Periodic Table Ppt Download
Q Tbn And9gcr5fzlxdbhkci O Kt Nierruqf0qmepfzuixr2cfjvysd2hbzg Usqp Cau
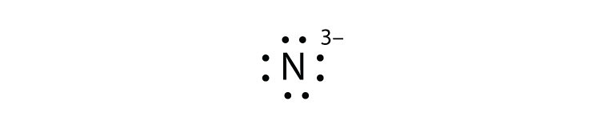
Lewis Electron Dot Diagrams Introductory Chemistry 1st Canadian Edition

Lewis Electron Dot Diagrams
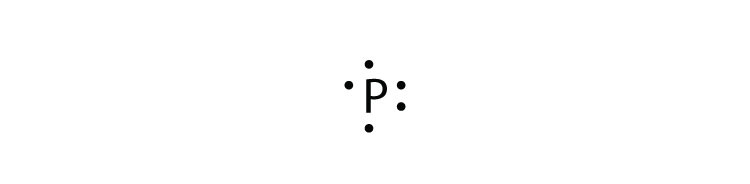
Lewis Electron Dot Diagrams

Argon Properties Uses Atomic Number Facts Britannica
How To Read The Periodic Table Overview Components Expii
The Elements Elements Are Pure Substances The Atoms Of Each Element Are Chemically Distinct And Different From Those Of Any Other Element Approximately 110 Elements Are Now Known By 1980 106 Of These Had Been Unequivocally Characterized And

Molecule Definition Examples Structures Facts Britannica
Kids Science Periodic Table Of Elements
Q Tbn And9gctml96yy77ecalx0sqdevvqznemvnbmp8y5fgzgoj8cr0vrkksf Usqp Cau

Valence Electrons Ck 12 Foundation

13 1 Dot Diagrams
Lewis Dot Structures Dots Are Arranged Around An Element S Symbol To Indicate The Valence Electrons Reminder Valence Shell Only Has S P Orbitals Maximum Ppt Download

Chemical Element Wikipedia

Atoms Molecules And Compounds Manoa Hawaii Edu Exploringourfluidearth

8 1 Chemical Bonds Lewis Symbols And The Octet Rule Chemistry Libretexts

Sulfur Definition Element Symbol Uses Facts Britannica
4 5 Lewis Dot And Bonding Chemistry Libretexts

Lewis Structures This Is Also Called The Electron Dot Structure Look At The Highest Energy Level Of An Atom Add The Number Of Electrons Within That Energy Ppt Download
Solved Bonding And Molecular Geometry Choose Three Compou Chegg Com

Glava 9 Br Section A Br Lewis Electron Dot Diagrams
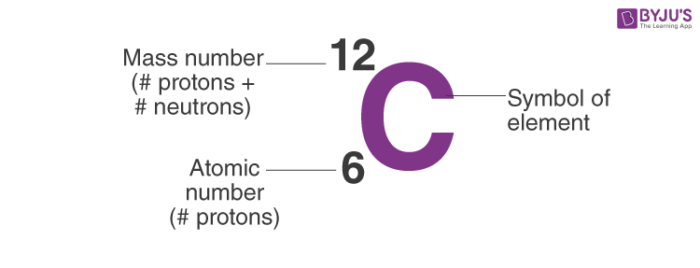
Atomic Number Mass Number And Isotopes Video Khan Academy

How To Read The Periodic Table Overview Components Expii
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library
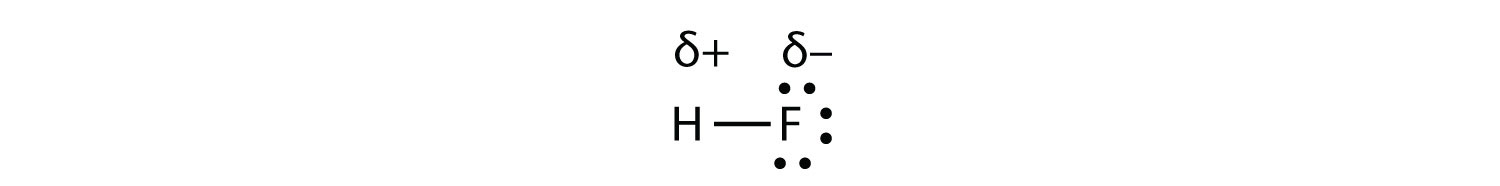
Chemical Bonds
Chemical Element Wikipedia

Periodic Table Definition Elements Groups Charges Trends Facts Britannica
Q Tbn And9gcq3udtzpei7a6xhrag2hmyphbhprepf61tdqeigrfejhoywl2vr Usqp Cau
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

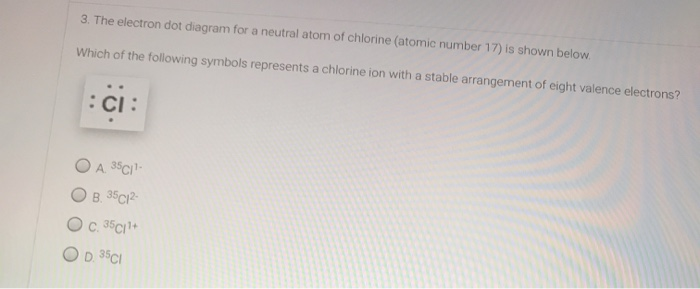
3 The Electron Dot Diagram For A Neutral Atom Of Chlorine Atomic Number 17 Is Shown Homeworklib
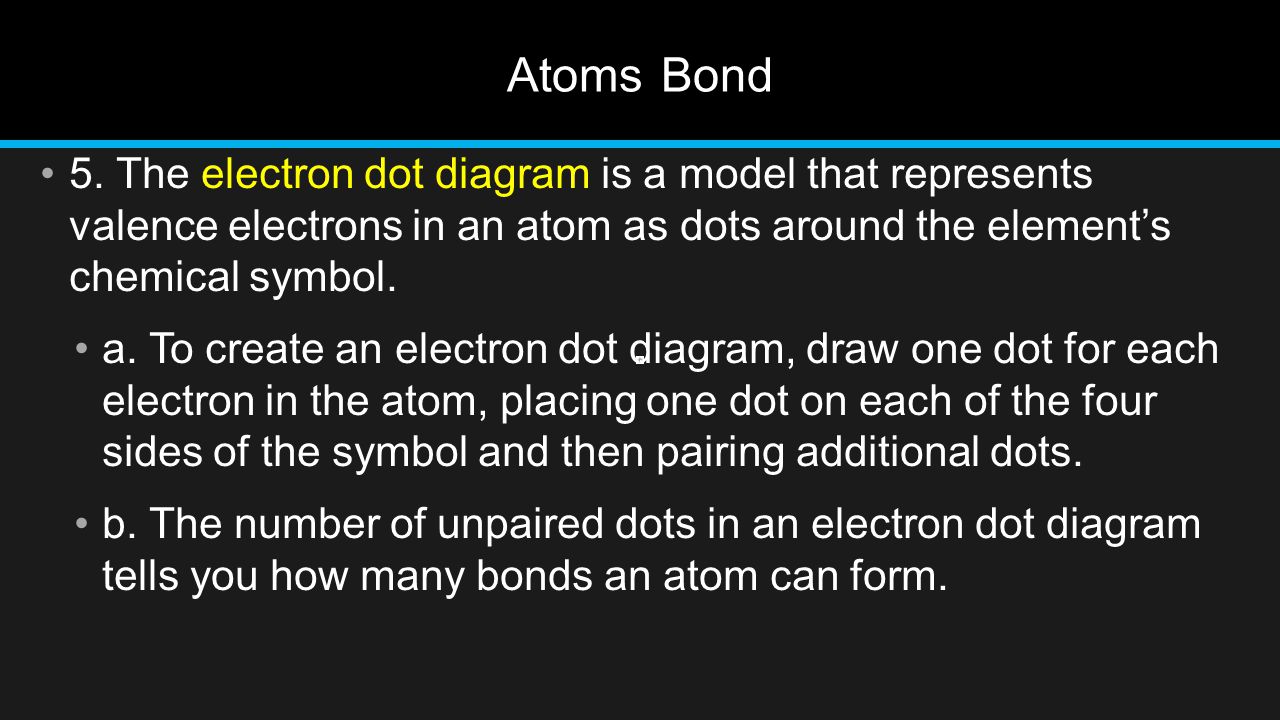
Chapter 8 Elements And Chemical Bonds Ppt Download
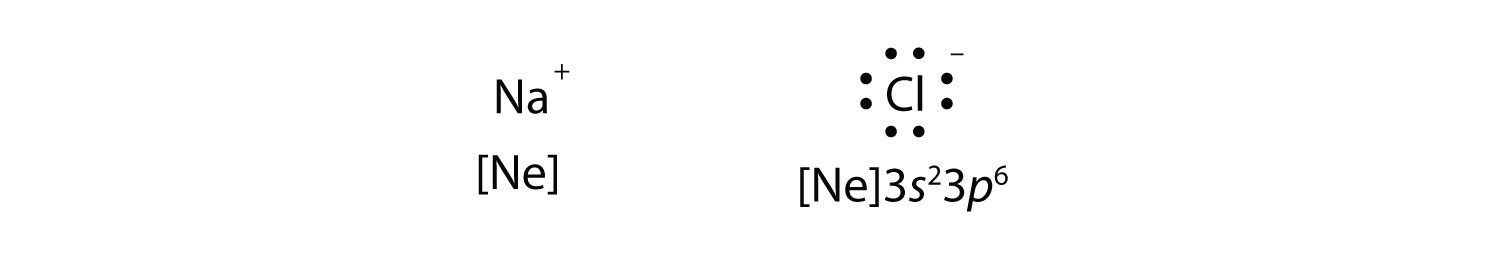
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library

Valence Electrons And Energy Levels Of Atoms Of Elements Video Lesson Transcript Study Com
Valence Electrons And Ionic Compounds Video Khan Academy
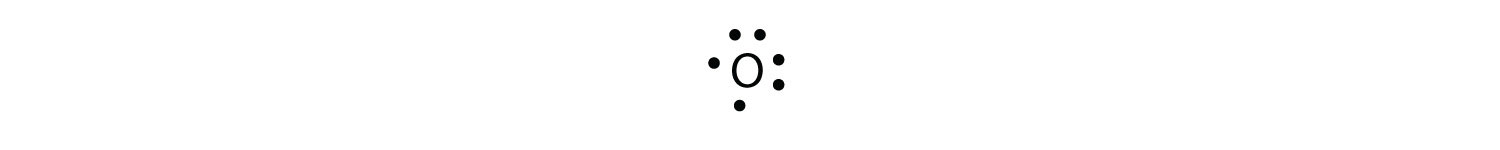
Lewis Electron Dot Diagrams

Chemistry I Atoms And Molecules
The 8 Types Of Arrows In Organic Chemistry Explained Master Organic Chemistry

Facts About Oxygen Live Science
/what-are-the-first-20-elements-608820-FINAL-5b758ab446e0fb002c67279a.png)
What Are The First Elements Names And Symbols
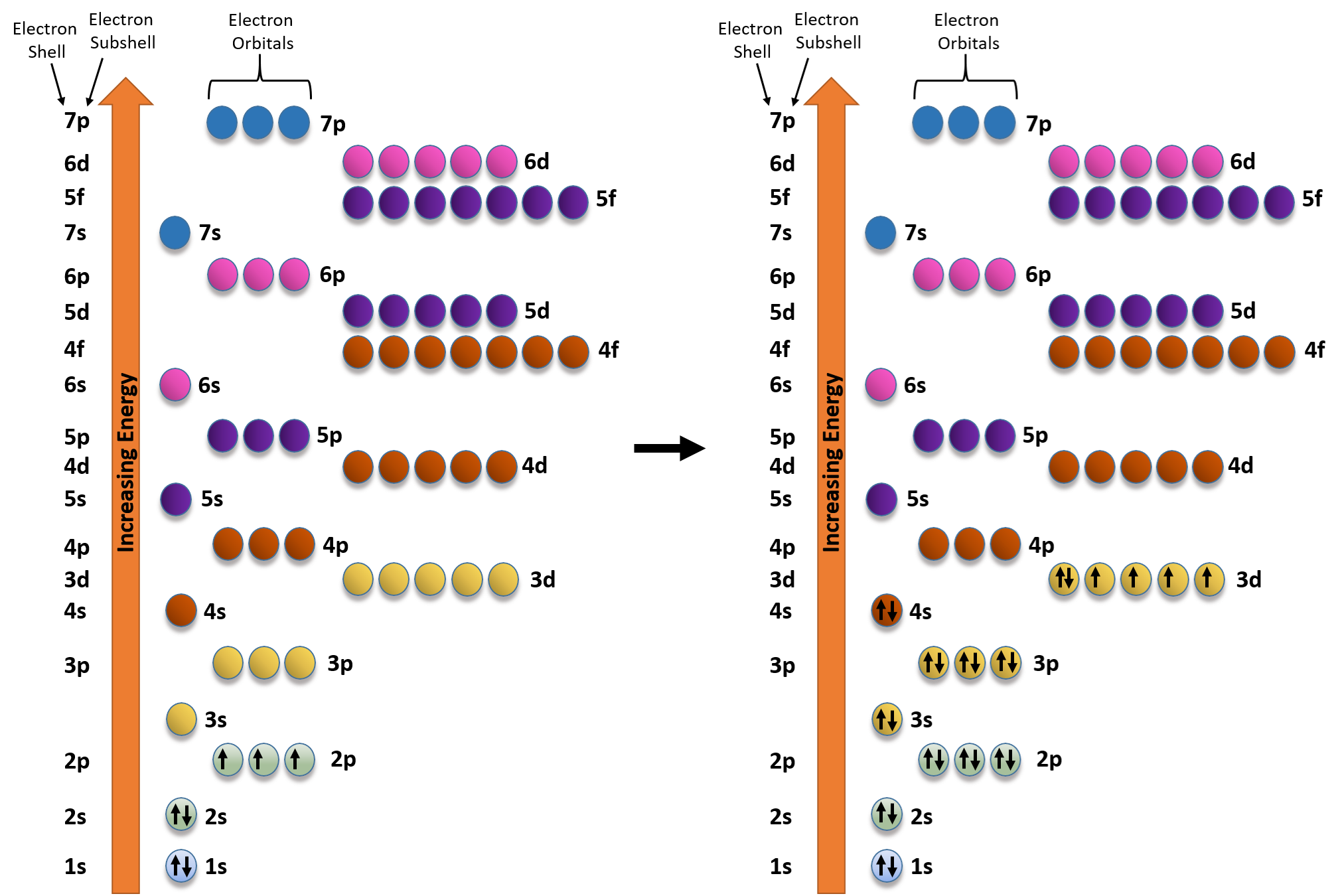
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

Lewis Dot Symbols And Lewis Structures Boundless Chemistry

Periodic Table Definition Elements Groups Charges Trends Facts Britannica



