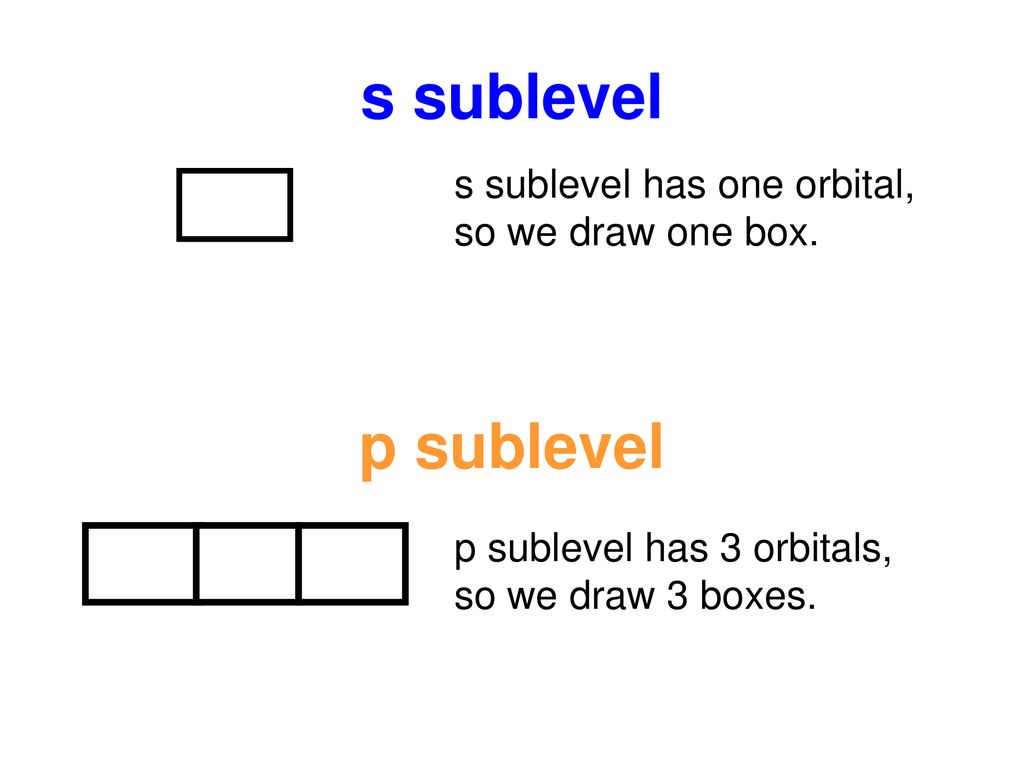
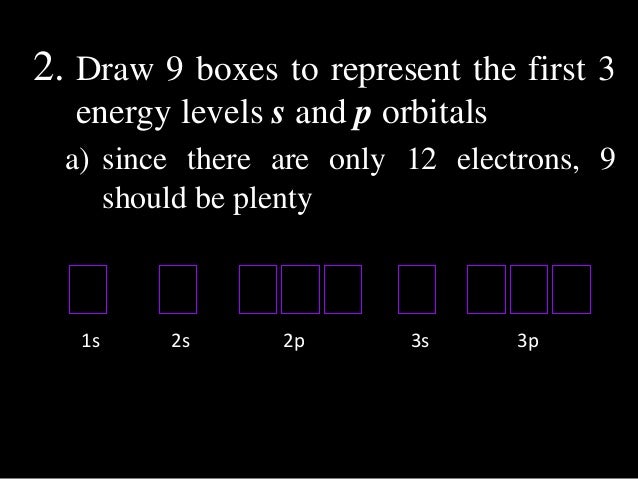
S Orbital Boxes
Http Ptfaculty Gordonstate Edu Lgoodroad Summer 11 Chem 1211 Chapter 8 solutions 5b1 5d Pdf

Chemistry I Atoms And Molecules

Ib Chemistry Topic 12 1 Electronic Configuration

Oneclass Write The Electron Configurations For P And Cl Using Both Spdf Notation And Orbital Box Dia
Www Birdvilleschools Net Cms Lib2 Tx Centricity Domain 3134 Electron configurations powerpoint Pdf
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
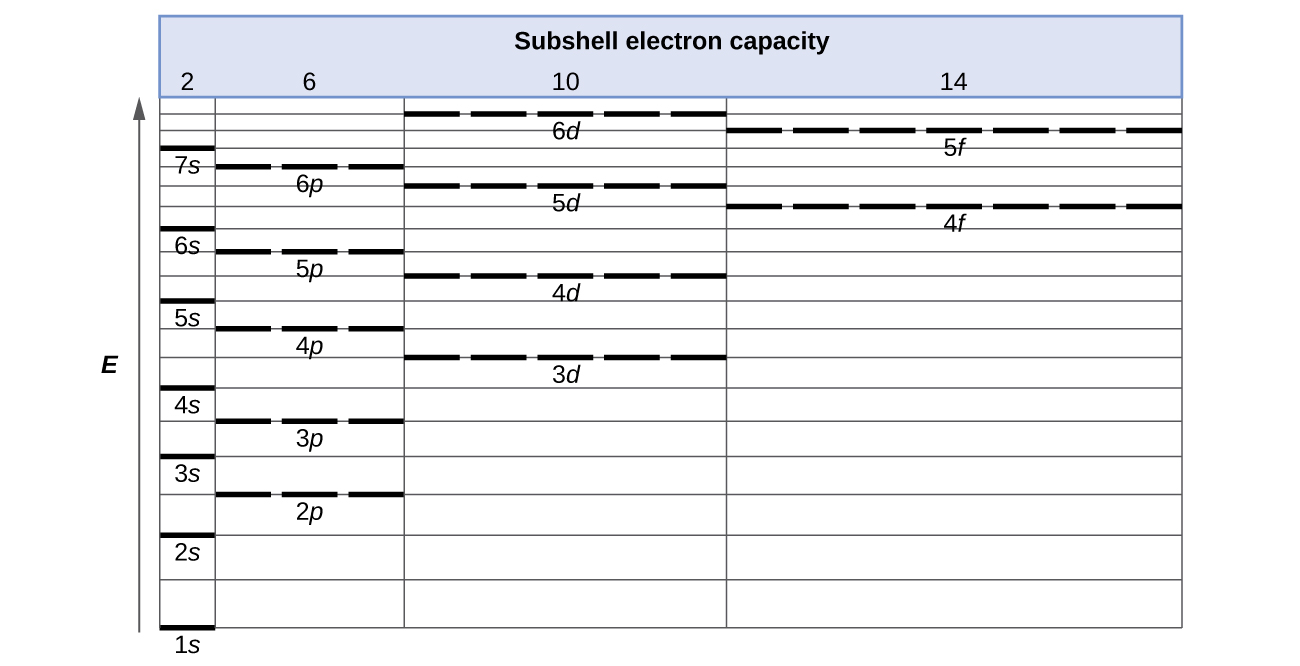
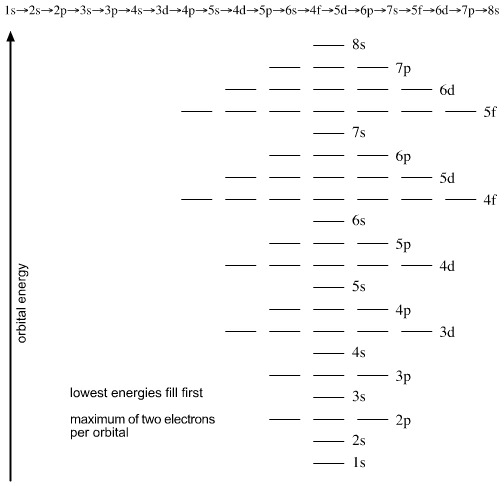
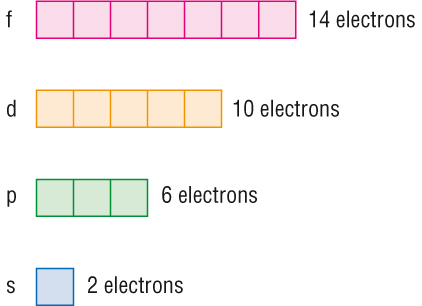
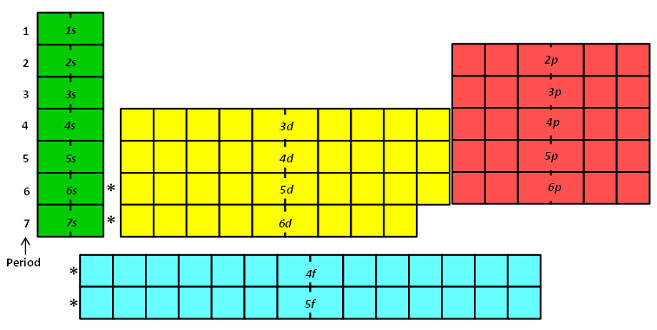
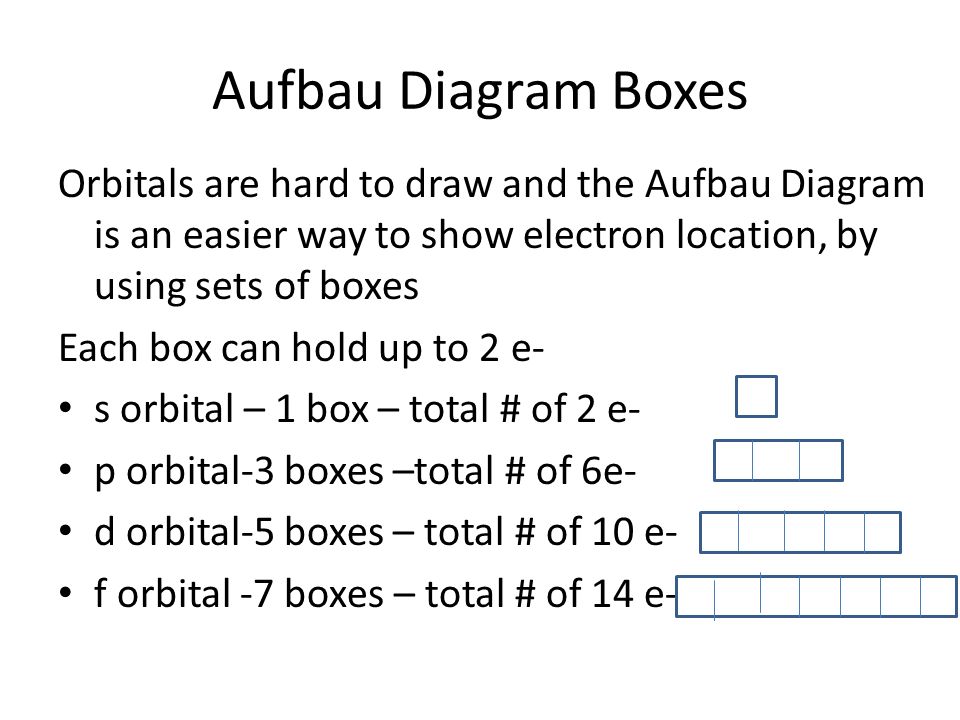
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f s can hold 2 electrons p can hold 6 electrons d can hold 10 electrons f can hold 14 electrons Note that individual orbitals hold a maximum of two electrons There can be two electrons within an s orbital, p orbital, or d orbital.
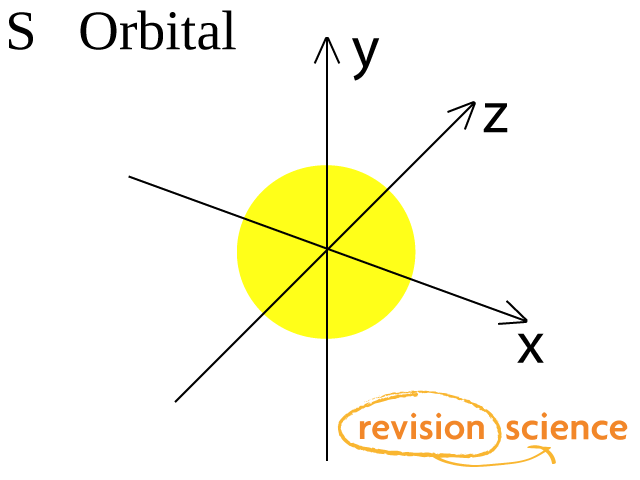
S orbital boxes. "The Box" is a single by the British electronica duo Orbital Taken from their 1996 album In Sides, the single was released in 1996 and reached number 11 on the UK Singles Chart. Aristotle, one of history’s most famous philosophers, said, “In all things of nature there is something of the marvelous” It turns out (perhaps unsurprisingly) that Aristotle was right Over the last few decades, a growing body of research has found that we would all do well to spend more time in the great outdoors. The orbital occupied by the hydrogen electron is called a 1s orbital The number "1" represents the fact that the orbital is in the energy level closest to the nucleus The letter "s" indicates the shape of the orbital s orbitals are spherically symmetric around the nucleus— they look like hollow balls made of chunky material with the nucleus at the center.
However the fact that its strength is added to the underlying σbond bond makes for a stronger overall linkage Electrons in πbonds. Everyone has to make their own decision but I do think Wolfe is the only way to go for orbital box osteotomy That's not a procedure I've realized I need, but I am having the malar osteotomy done by him If anyone wants to wait a year and a half till I have it done, wait by all means, but you'd just be wasting your time since your mileage may. Steering Gear Boxes & Orbitals JavaScript seems to be disabled in your browser For the best experience on our site, be sure to turn on Javascript in your browser.
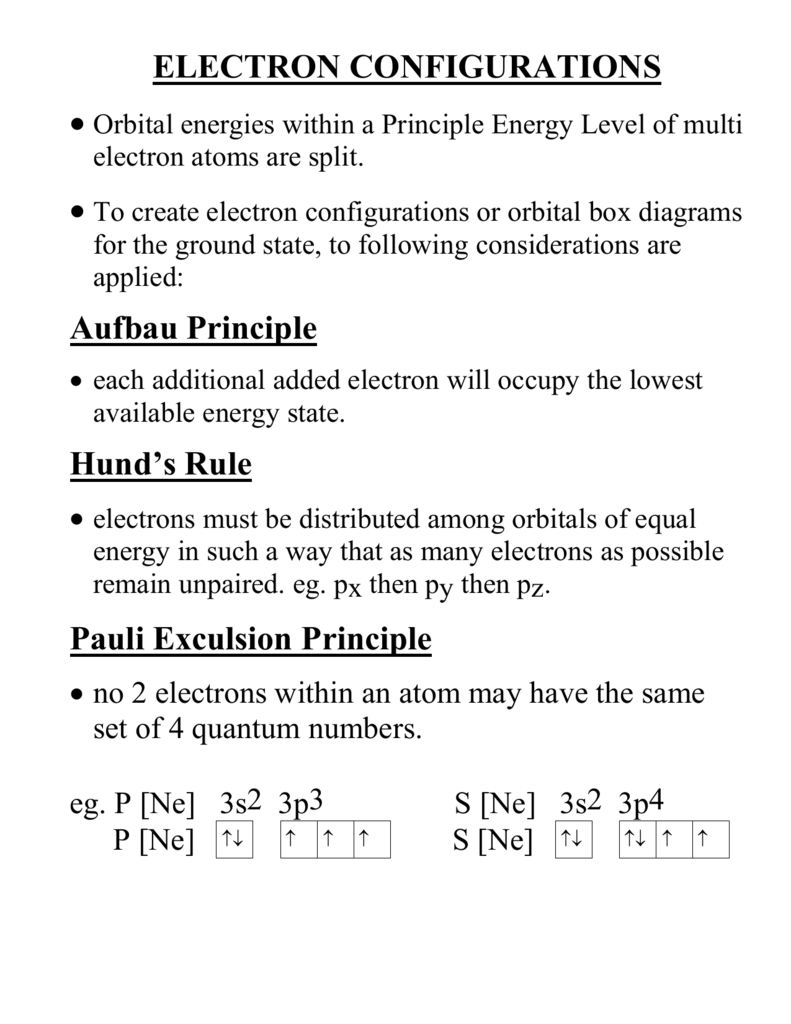
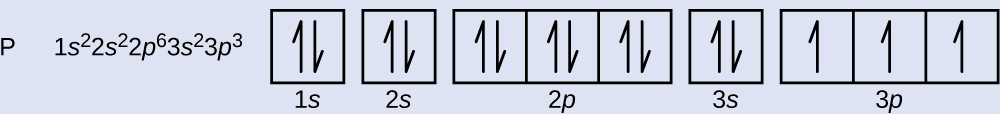
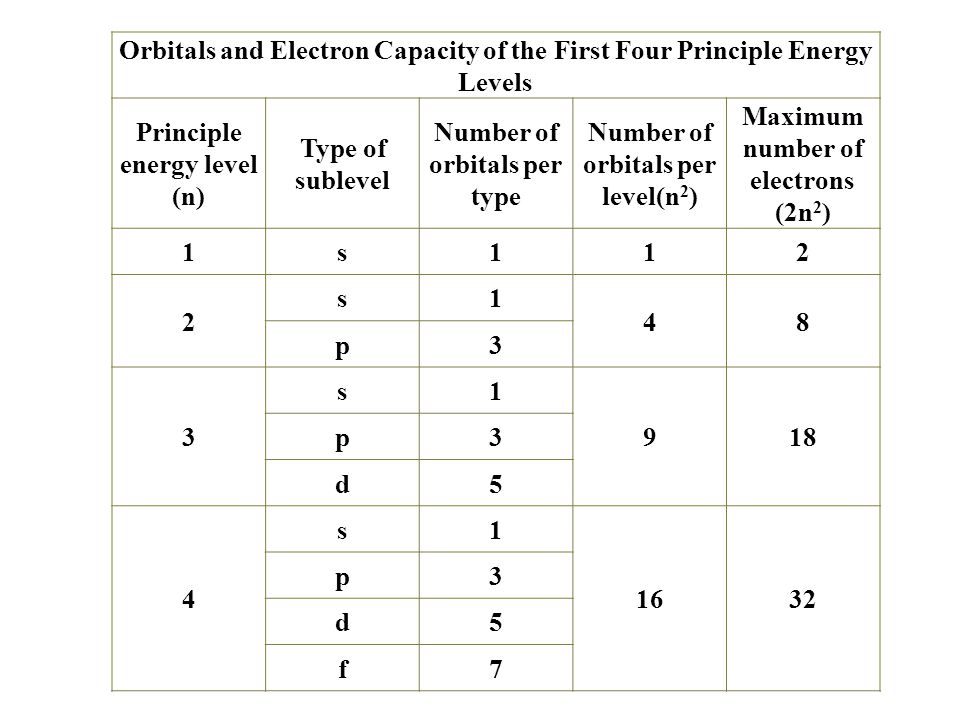
1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons) When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The number of orbitals in each sublevel vary with s contain one, p containing three, d containing five, and f containing seven Each orbital can only hold two electrons.
2 The lobes of a p orbital disappear at the nucleus What does this tell us about electrons in p orbitals?. What is S Orbital S orbital is an atomic orbital that has a spherical shape It has the lowest energy when compared to other atomic orbitals Each electron shell has at least one s orbital S orbital is the simplest atomic orbital among other orbitals One s orbital can hold a maximum of two electrons S orbitals have no suborbitals. The probability of finding an electron at the nucleus is 0 (you will never find an electron in the nucleus) 3 2The electron configuration for phosphorus, written in core notation, is Ne 3s 3p 3 What two things does Hund’s rule tell us.
"The Box" is a single by the British electronica duo Orbital Taken from their 1996 album In Sides, the single was released in 1996 and reached number 11 on. In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons) When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. This video explains s, p, d, and f orbitals, sublevels, and their shapes It discusses the 4 quantum numbers n, l, ml, and ms n represents the energy leve.
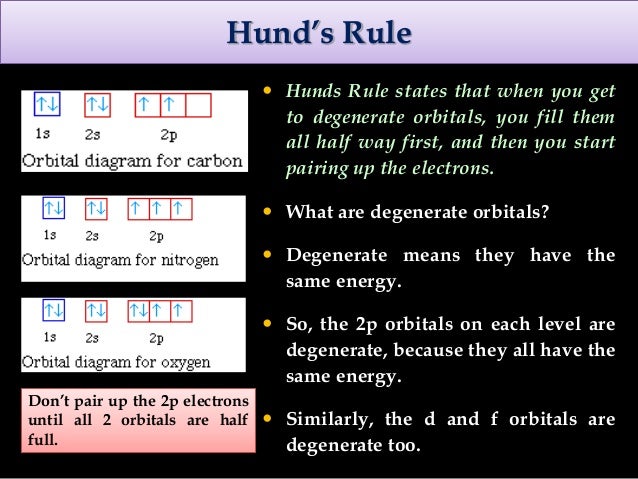
S ORBITALS An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger The order of size is 1s < 2s < 3s < , as shown below. * The Orbital Rotating Rubber Pin Box is not compatible with Curt Q, Dodge M, Gooseneck hitches and couplers like Andersen Ultimate and Pullrite Superlite OPTIONS Pin Box for 11,500 lbs or less GVWR;. Orbital diagram (orbital box diagram) Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and three of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.
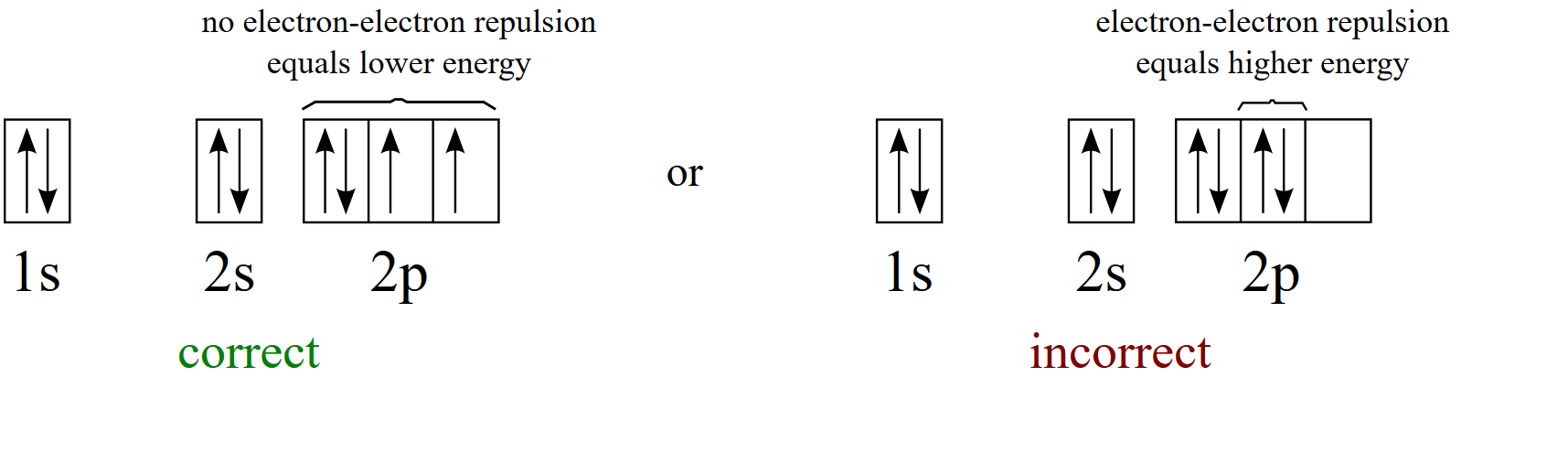
According to Hund’s rule, as electrons are added to a set of orbitals of equal energy, one electron enters each orbital before any orbital receives a second electron Orbital Filling Diagrams An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental) Within each shell of an atom there are some combinations of orbitals In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4 up shells you find all four types of orbitals. Orbital diagram (orbital box diagram) Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, and 4s orbitals, with 2 electrons occupying 2 of the 3d orbitals, so we apply Hund's Rule to maximise the number of unpaired electrons and give them parallel spin This means that 2 of the 3d orbitals will be occupied by 1 electron and these two arrows will point in the same direction.
Fill orbitals in the first energy level (1s) Orbital box notations provide information about the number of paired and unpaired electrons in an atom, and that information can be used to determine whether the atoms are paramagnetic or diamagnetic Hydrogen has one unpaired electron and is a paramagnetic species, whereas helium’s. 7 The d orbital starts in the 4th row, or 4th energy level. Orbital This is a common picture of a p x orbital This simplifi ed p x orbital is often useful A hand drawn version does not have to be exact Use this box to draw a p z orbital We can combine all three p orbitals in a three dimensional display y x z y x z Use these axes to draw all three p orbitals ORBITALS AND MOLECULAR REPRESENTATION 3.
This is called a node or nodal surface In 2s orbital there is one spherical node The number of nodal surfaces or nodes in sorbital of any energy level is equal to (n1), where n is the principal quantum number Shape of porbitals For psubshell l = 1, there are three values of m namely 1, 0, 1 It means that p orbitals can have three possible orientations. There is yet another way to writing electron configurations It is called the "Box and Arrow" (or circle and X) orbital configuration Sublevels can be broken down into regions called "orbitals" An orbital is defined as the most probable location for finding an electron Each orbital holds 2 electrons. Steering Gear Boxes & Orbitals JavaScript seems to be disabled in your browser For the best experience on our site, be sure to turn on Javascript in your browser.
They are hybridized atomic orbitals formed by mixing s and p orbitals, to describe bonding in molecules In an sp^3 hybridization, color(red)"one" s orbital is mixed with color(red)"three" p orbitals to form color(red)"four" sp^3 hybridized orbitals Each of these hybridized orbitals have 25% s character and 75% p character (calculated according to the proportion of sp mixing). Owner's manual wedge guide options Rubber Pin Box FEATURE PRODUCTS learn more THPEX2 PatioEX. Didn't Read) Electron configurations have the format 1s 2 2s 2 2p 6The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number;.
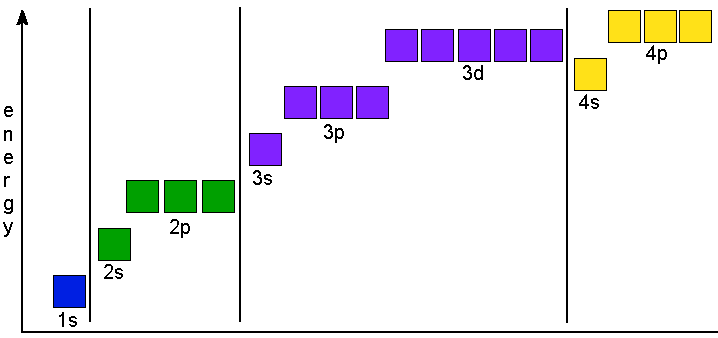
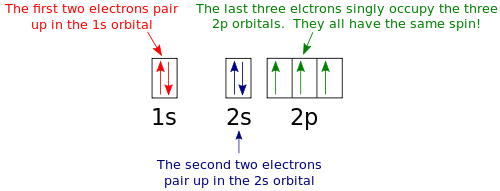
According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium The next element is lithium and necessitates the use of the next available sublevel, the 2s. Orbital This is a common picture of a p x orbital This simplifi ed p x orbital is often useful A hand drawn version does not have to be exact Use this box to draw a p z orbital We can combine all three p orbitals in a three dimensional display y x z y x z Use these axes to draw all three p orbitals ORBITALS AND MOLECULAR REPRESENTATION 3. PHOTOS Looking Back On The Ravens' Win At Super Bowl 47The Baltimore Ravens defeated the San Francisco 49ers at Super Bowl 47 in a wild 3431 victory that was held up by a power outage at the.
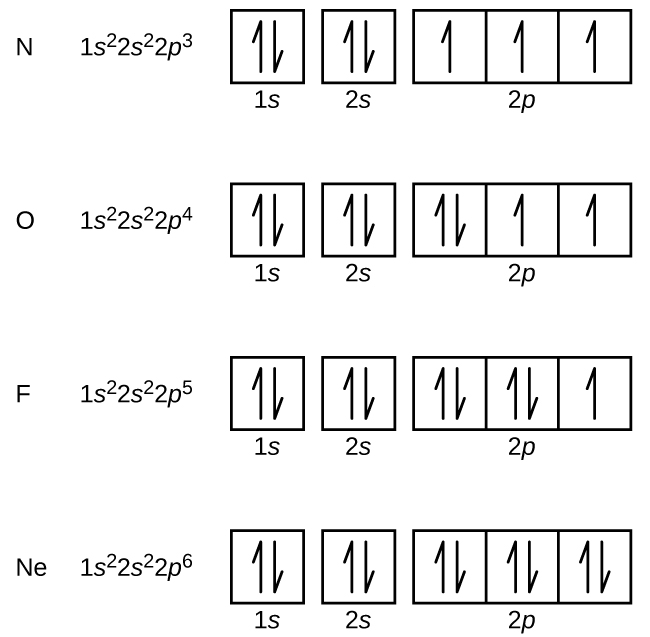
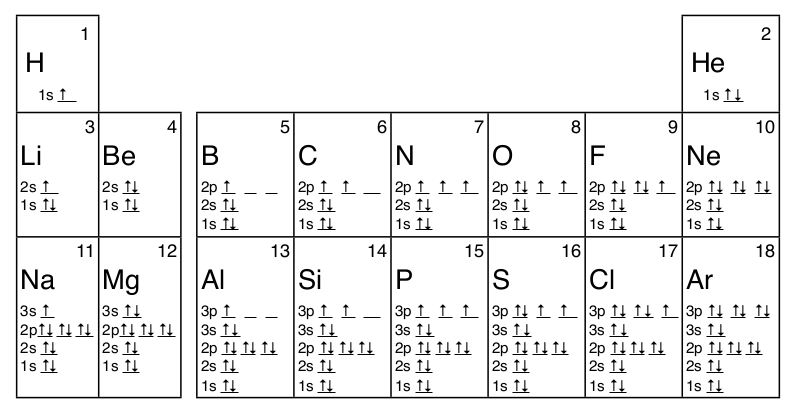
The proposed technique results in 256 different Sboxes named as orbital Sboxes Rigorous tests and comparisons are performed to analyse the cryptographic strength of each of the orbital Sboxes. The diagram shows the number of subshell by using boxes or lines for electrons (use three for porbitals, five for dorbitals, and 7 for forbitals) In each box the spin of an electron is noted by using arrows, up arrows mean 1⁄2 spin and down arrows mean –1⁄2 spin For example, the orbital diagram for the first 18 atoms are shown below. The "1" represents the fact that the orbital is in the energy level closest to the nucleus The "s" tells you about the shape of the orbital s orbitals are spherically symmetric around the nucleus in each case, like a hollow ball made of rather chunky material with the nucleus at its centre The orbital on the left is a 2s orbital.
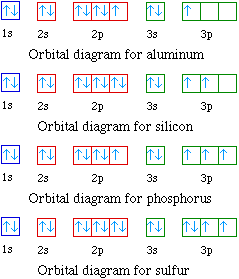
Porbital overlap is less than headon overlap between two s orbitals in a σbond due to orbital orientation This makes the πbond a weaker bond than the original σbond that connects two neighboring atoms;. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins The boxes represent sulfur's orbitals Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Large Storage Bins Baskets 3 pack 157x118x”Foldable Sturdy Storage Basket with Handle,Decorative Storage Boxes for Shelves, Rectangle Closet Baskets Box, Organizing Nursery for HomeOffice 47 out of 5 stars 2,580.
Fred Senese of Antoine Frostburg explains "You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do with. One of the four hybrid orbitals formed by hybridization of an s orbital and three p orbitals Note that the total electron density changes (ie, the shape of the orbital changes) as the amounts of s and p character for the orbital change. Sublevel s contains one orbital, p contains three, d has five, f has seven, g has nine, h has 11 and i has 13 The periodic table of elements contains seven rows;.
We need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals. Therefore, elements can have up to seven energy levels, depending on how many electrons the element possess Electrons travel around the atom in orbitals. In atomic theory and quantum mechanics, an atomic orbital is a mathematical function describing the location and wavelike behavior of an electron in an atom This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleusThe term atomic orbital may also refer to the physical region or space where the electron can be.
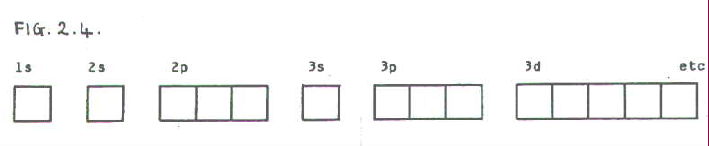
The diagram shows the number of subshell by using boxes or lines for electrons (use three for porbitals, five for dorbitals, and 7 for forbitals) In each box the spin of an electron is noted by using arrows, up arrows mean 1⁄2 spin and down arrows mean –1⁄2 spin For example, the orbital diagram for the first 18 atoms are shown below. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Orbitals can be represented as boxes with the electrons in them shown as arrows Often an uparrow and a downarrow are used to show that the electrons are in some way different A 1s orbital holding 2 electrons would be drawn as shown on the right, but it can be written even more quickly as 1s 2 This is read as "one s two" not as "one s squared".
The lithium 1s orbital is the lowestenergy orbital on the diagram Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding;. What is S Orbital S orbital is an atomic orbital that has a spherical shape It has the lowest energy when compared to other atomic orbitals Each electron shell has at least one s orbital S orbital is the simplest atomic orbital among other orbitals One s orbital can hold a maximum of two electrons S orbitals have no suborbitals. Look at the periodic table above, all the orange boxes indicates the principle energy level and the atomic orbital that the outer electrons in that specific element occupies 6 Look at Helium (He), is it part of the “s” orbital or “p”orbital?.
Due to the size of the orbital files, it may take several seconds for the orbitals to appear, only the total electron density is shown for each orbital (ie, the phases for each orbital are not shown), and when a hybrid orbital is depicted in a textbook, the shape of the orbital is often exaggerated (elongated) to illustrate the directionality of the orbital along a particular axis, or.
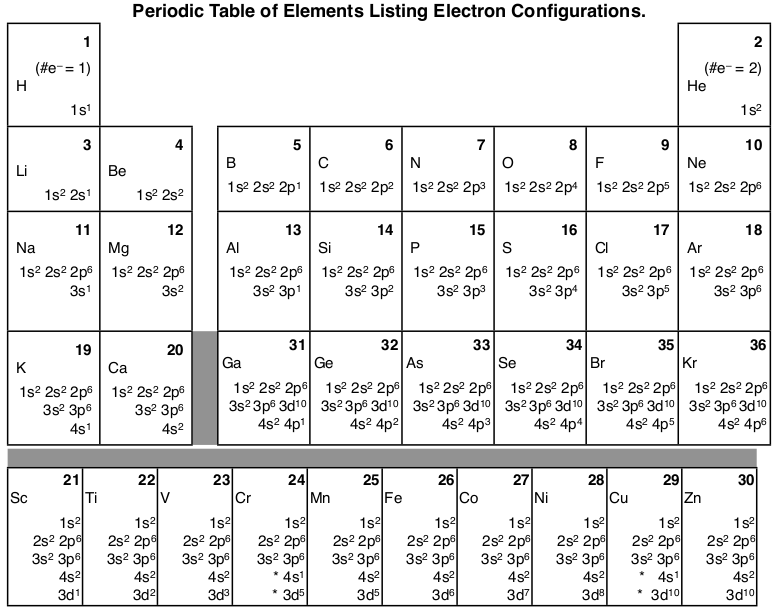
Silicon Electron Configuration Si With Orbital Diagram
2
Www Gsjournal Net Science Journals Research papers Chemistry Download 7073
Electronic Structure Of Atoms Electron Configurations Chemistry 2e
Group Activity Electron Configuration

Orbital Diagrams Ppt Electron Configuration Atomic Orbital
Quantum Numbers To Periodic Tables Chemogenesis
Solved 5 What Do You Notice About The Orientations And Th Chegg Com
Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its Electron Configuration
Chembook Co Uk Chemistry In Perspective For Bored And Confused Senior School Students

Electronic Configuration The Atom Siyavula
Electronic Structure Of Atoms Electron Configurations Chemistry
Q Tbn And9gcrjaqjlb8lpy2zmnxb7djnungtx4z3mbsdjg7v7iofb5bmcmoq Usqp Cau
Http Mrsslovacek Weebly Com Uploads 5 8 2 5 Orbital Filling Diagrams And Electron Configuration Practice 2 Key 16 Pdf

Orbital S Are Represented By Boxes Grouped By Sublevel W Small Arrows Indicating The Electrons
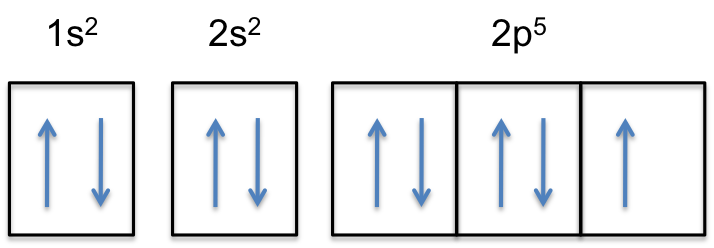
What Is The Electron Configuration Of F Socratic
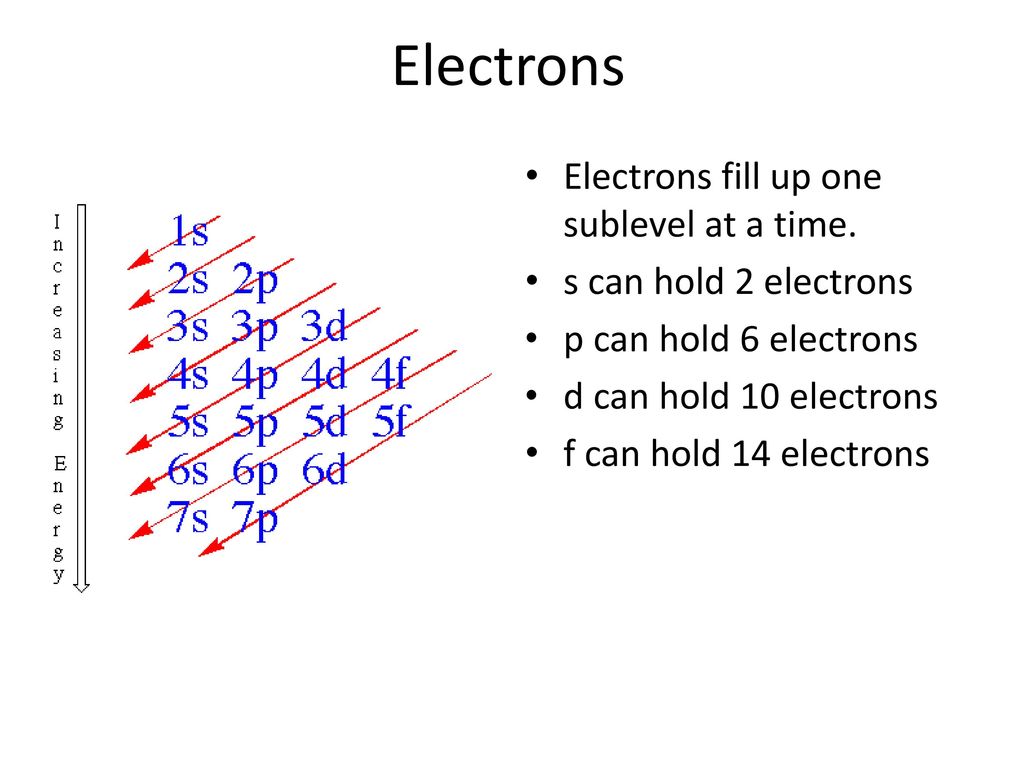
Electrons Orbitals Ppt Download

Orbital Configurations Ck 12 Foundation

Chemistry The Central Science Chapter 6 Section 8
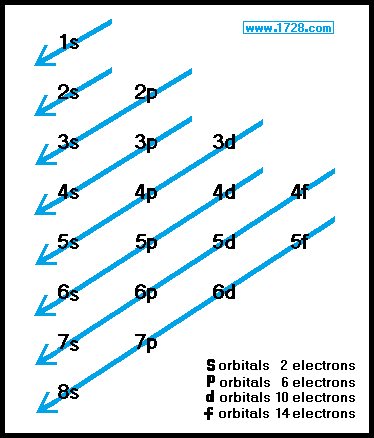
Orbitals And Electron Configuration
Www Chem Uwec Edu Chem103 F08 F0f Pages Lecture Materials Unit Ii Lecture 8 Publishers Overheads Unit Ii Lecture 8 Publishers Overleads Pdf
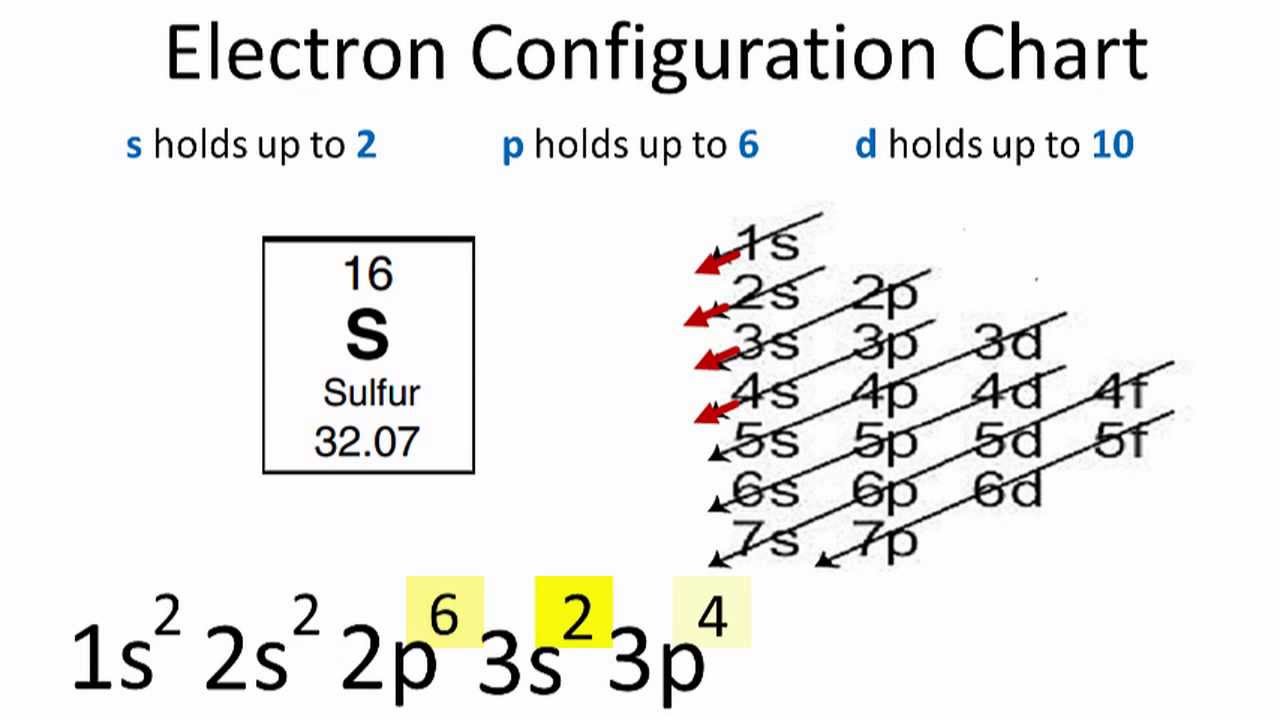
Electron Configuration For Sulfur S

5 5orbitaldiagrams 1 Pdf Orbital Diagrams Name Chem Worksheet 5 5 An Orbital Diagram Uses Boxes With Arrows To Represent The Electrons In An Atom Each Course Hero

Atomic Electron Configurations

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Electron Configuration Wyzant Resources
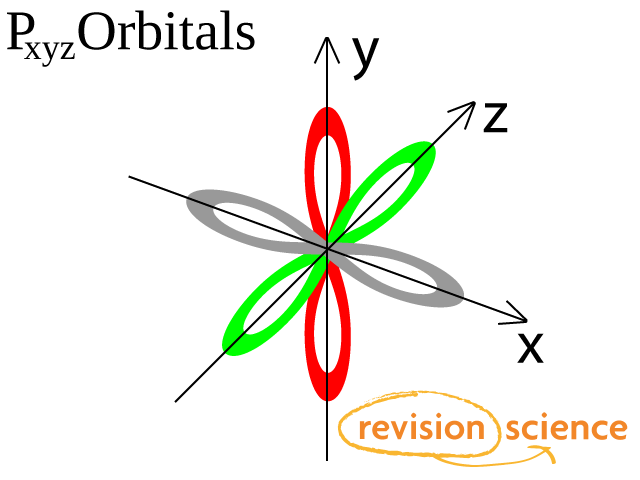
Orbitals A Level Chemistry
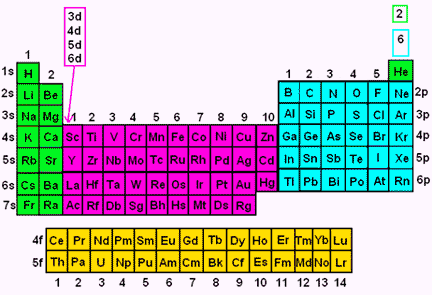
Electron Configuration Wyzant Resources
Q Tbn And9gctx2unihrwvsl45ij5h Bp2grxrgaokdparonvakfmrfmh9cyz4 Usqp Cau

Electron Configuration Worksheet Easy Hard Science
Solved Draw Orbital Diagrams Boxes With Arrows I
Electron Configurations And Boxes Sch4u1 Ccvi
Q Tbn And9gcth1rc3hbnde1titk095wzz5fdzyo5obndscg8azgis25 Lq4re Usqp Cau
If Each Orbital Can Hold A Maximum Of 3 Electrons The Number Of Elements In The 4th Period Of The Periodic Table Long Form Is Socratic

4 Give The Orbital Box Diagram For Ground State Configurati Clutch Prep

Electron Configurations
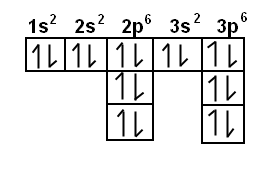
Electron Configuration Wyzant Resources

Electron Orbital Diagrams Electron Configuration Teaching Chemistry Chemistry Lessons
Electron Configuration Texas Gateway
Web Gccaz Edu Kimld531 Rev130 Files Wkstatomic Pdf
Www Austincc Edu Mohan Documents Ch08 Lecture 6e Final 000 Pdf

Pdf S Boxes Based On Affine Mapping And Orbit Of Power Function

Electron Configuration Boundless Chemistry

Orbital Diagrams Electron Configurations For Atoms And Ions

Schematic Representation Of Hybridization States Using Quantum Boxes Download Scientific Diagram

Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its Electron Configuration

S P D F Orbitals Explained 4 Quantum Numbers Electron Configuration Orbital Diagrams Youtube
Course S4 Chemistry Topic Unit 2 Electronic Configuration Of Atoms And Ions

Double And Triple Covalent Bonds Double Covalent Bonds

S P D F Obitals Notation Shapes Diagrams How To Work Out Electron Arrangements Configurations Order Of Filling Quantum Levels Electronic Structure Of Atoms Gce A Level Revision Notes

Electronic Configuration The Atom Siyavula
Orbital Diagrams Ppt Download
What Is The Orbital Configuration For Sulfur Quora
Www Unf Edu Michael Lufaso Chem45 Chapter6 Pdf

Solved 5 4 Pts A Write The Complete Electron Configur Chegg Com
1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts
3 1 Electron Configurations Chemistry Libretexts
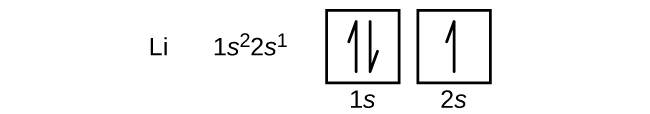
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry

Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
Electronic Configuration Final

Electron Configuration For Sulfur S
Www Graftonps Org Site Handlers Filedownload Ashx Moduleinstanceid 67 Dataid 113 Filename Review and critical thinking electron configuration and orbitals Pdf

Hund S Rule And Orbital Filling Diagrams Chemistry For Non Majors

Orbitals Flashcards Quizlet

5 3 Electron Configuration Flashcards Quizlet
Electron Configurations

Electron Configurations Of The 3d Transition Metals Video Khan Academy
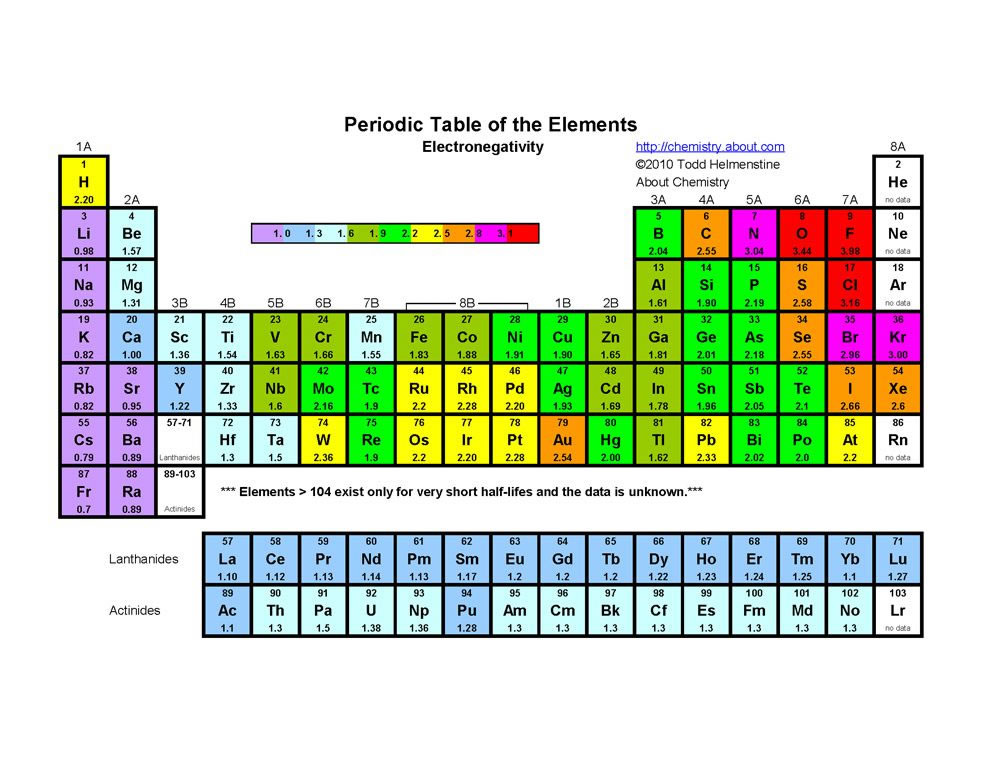
The Periodic Table In A Previous Section The Periodic Table Was Introduced As A List Of The Elements We Also Pointed Out That The Design Of The Periodic Table Separates The Metals From The Nonmetals In This Section We Will Show How The Various Features Of The

Is The Order Of Orientation Of Electron Box Diagrams Meaningful Or Arbitrary Chemistry Stack Exchange

Electron Configurations The Periodic Table

3 2 Electron Configuration Orbital Diagrams Piersonchemistry

A Level Chemistry Electronic Configuration Chemactive Com
Sigma Pi Bonding Atomic Orbital Bonding Sigma S Pi P Bonds
Orbitals A Level Chemistry
Q Tbn And9gcsj4fgjpix3utxsz25hefqhut0jqwxk8hf0 Vlozplqv8ginn Usqp Cau
Electronic Configuration Final
1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts

Electron Orbital Diagrams Electron Configuration Teaching Chemistry Chemistry Lessons

How To Represent Electrons In An Energy Level Diagram Dummies

The Periodic Table In A Previous Section The Periodic Table Was Introduced As A List Of The Elements We Also Pointed Out That The Design Of The Periodic Table Separates The Metals From The Nonmetals In This Section We Will Show How The Various Features Of The
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
High School Chemistry Orbital Configurations Wikibooks Open Books For An Open World

Orbital Configurations Ck 12 Foundation

Draw The Atomic Orbital Diagram For Chlorine

Electron Configurations For The Second Period Video Khan Academy

Box And Arrow Configurations Using Pauli Exclusion Principle And Hund S Rule
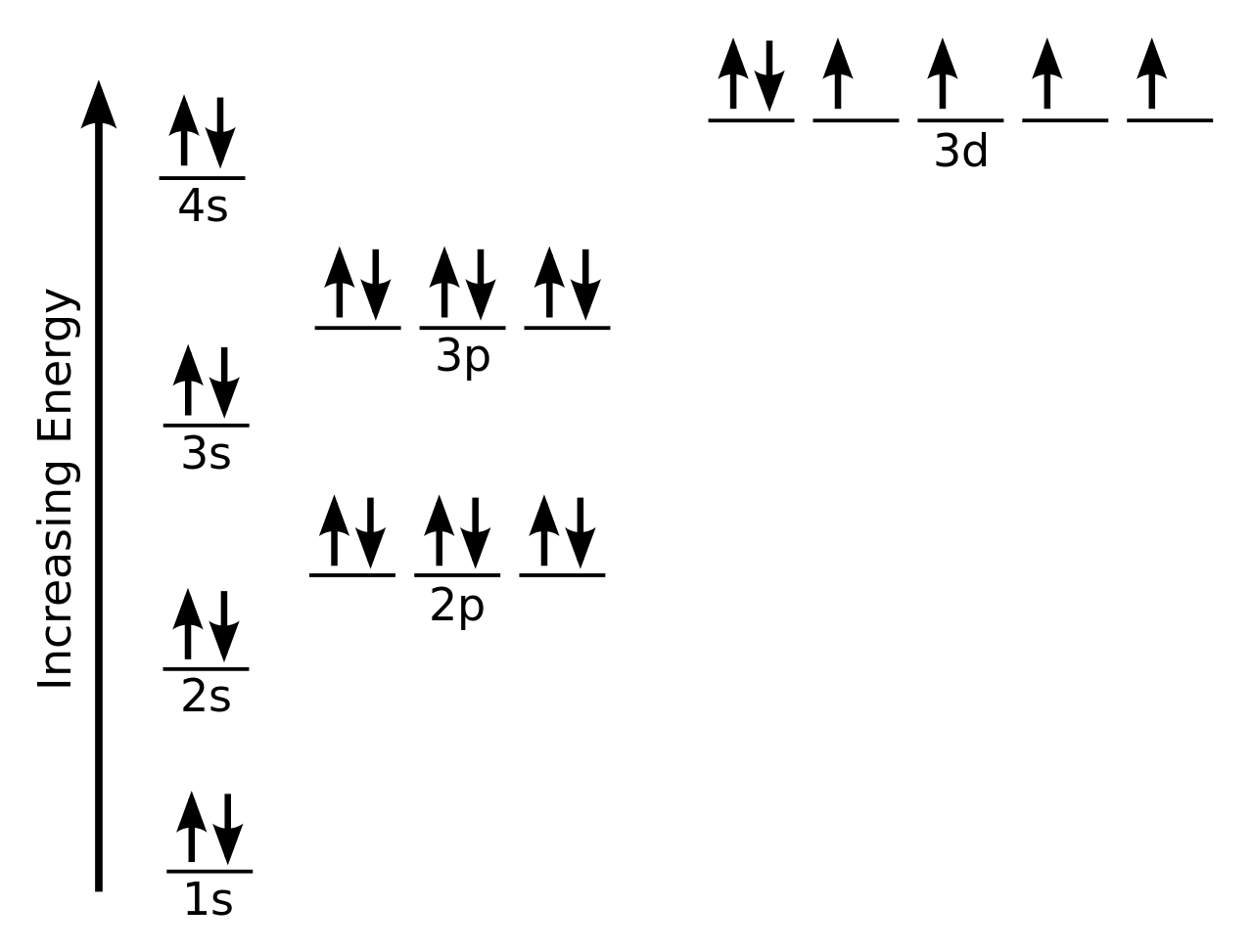
The Order Of Filling 3d And 4s Orbitals Chemistry Libretexts

Electron Configuration Wikipedia

Electron Orbital Diagram Of Vanadium Chemistry Stack Exchange
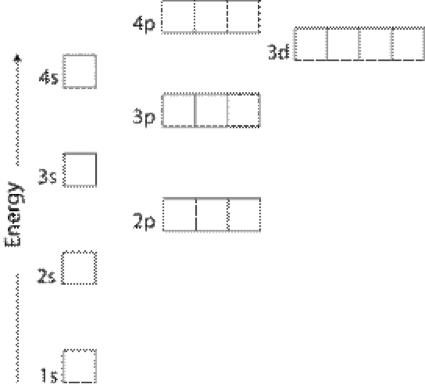
Electron Configurations Orbitals Energy Levels And Ionisation Energy Trends A Level Chemistry Revision Notes

How To Write Orbital Notation Youtube
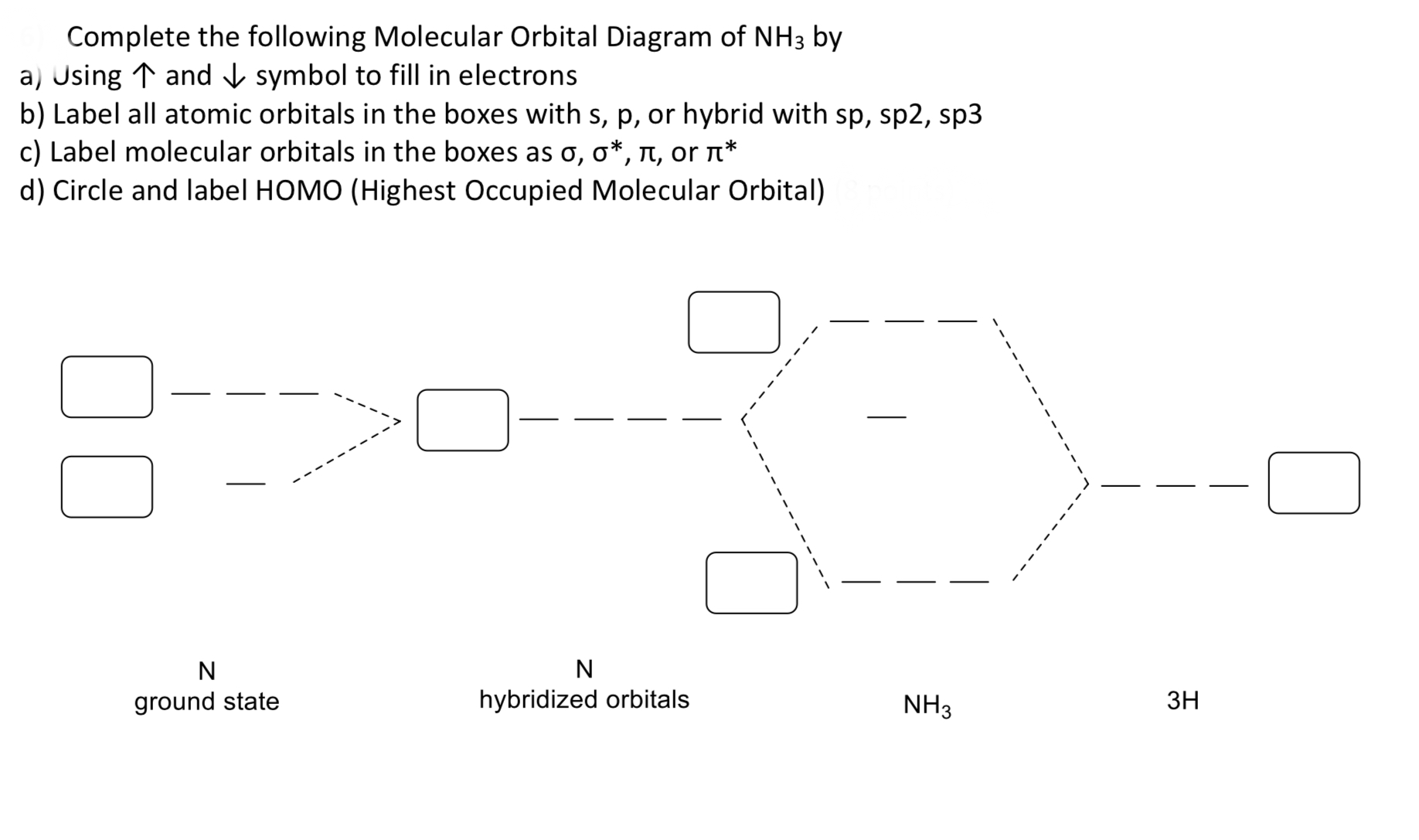
Answered Complete The Following Molecular Bartleby
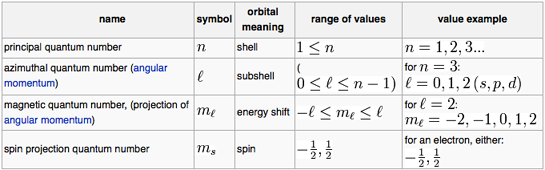
Rasayan Nuclear
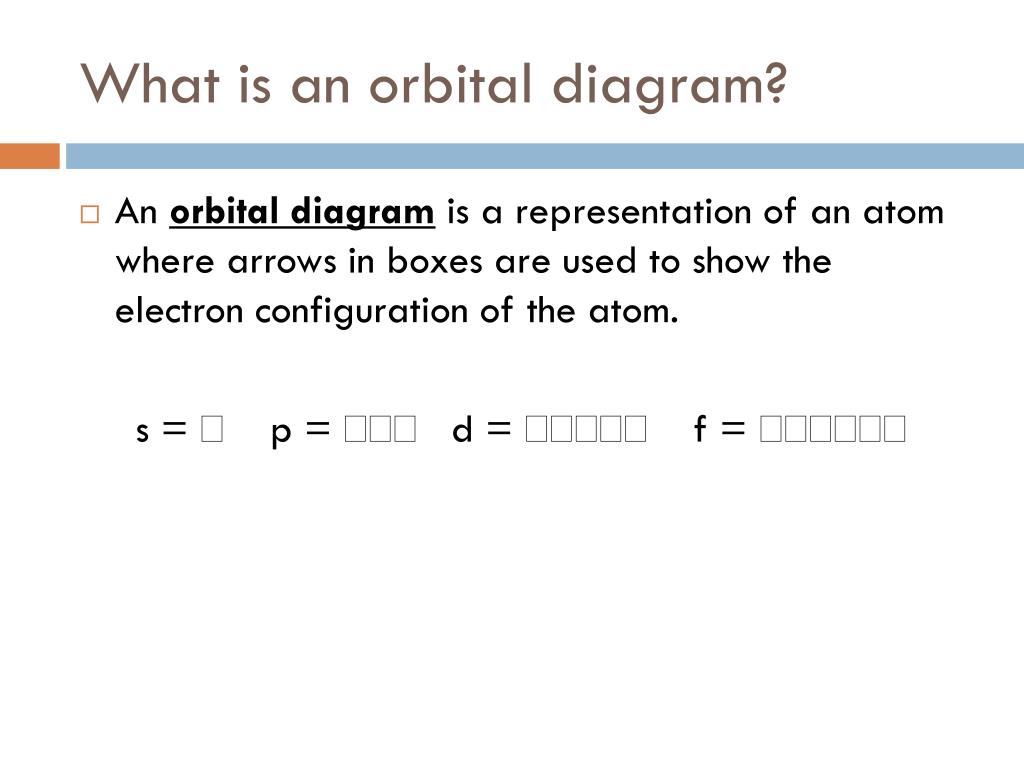
Ppt Orbital Diagrams Powerpoint Presentation Free Download Id
Electron Configurations Course Hero
Lecture 5 3 Aufbau Diagrams Ppt Download

Electron Configurations

A Level Chemistry Electronic Configuration Chemactive Com



