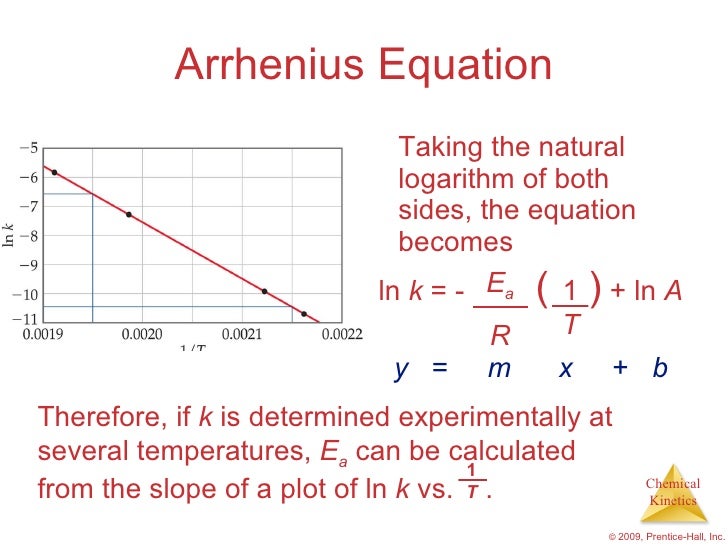
Arrhenius Equation Ymx+c

Arrhenius Equation The Effect Of Temperature On Reaction Rate

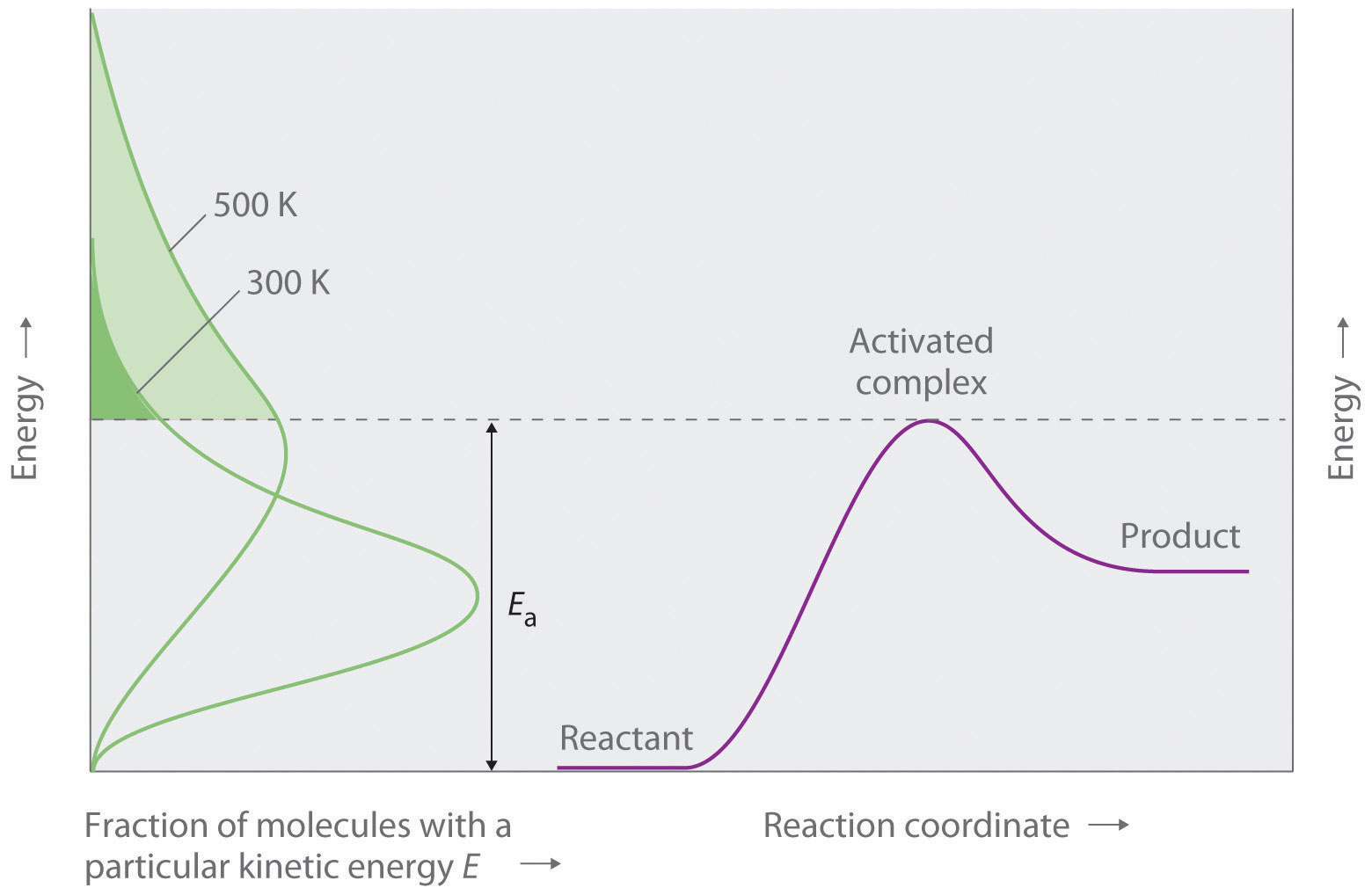
Ib Chemistry Ellesmere College 16 2 Activation Energy
According To Arrhenius Equation Rate Constant K Is Equal To Ae Ea Rt Studyrankersonline

Arrhenius Equation The Effect Of Temperature On Reaction Rate

19 Activation Energy Arrhenius Equation Ap Chemistry Educator Com
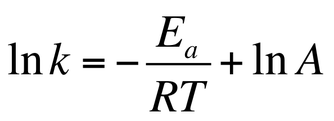
Track And Field Kinetics Ap Chemistry Olympics
B) What is the intercept?.
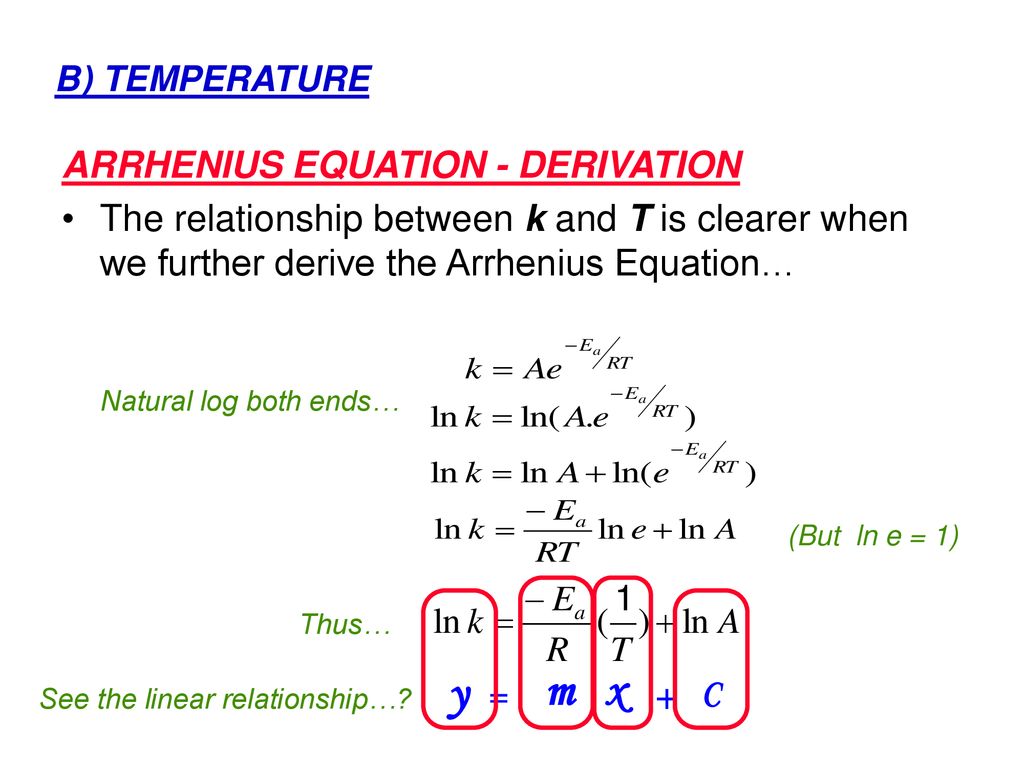
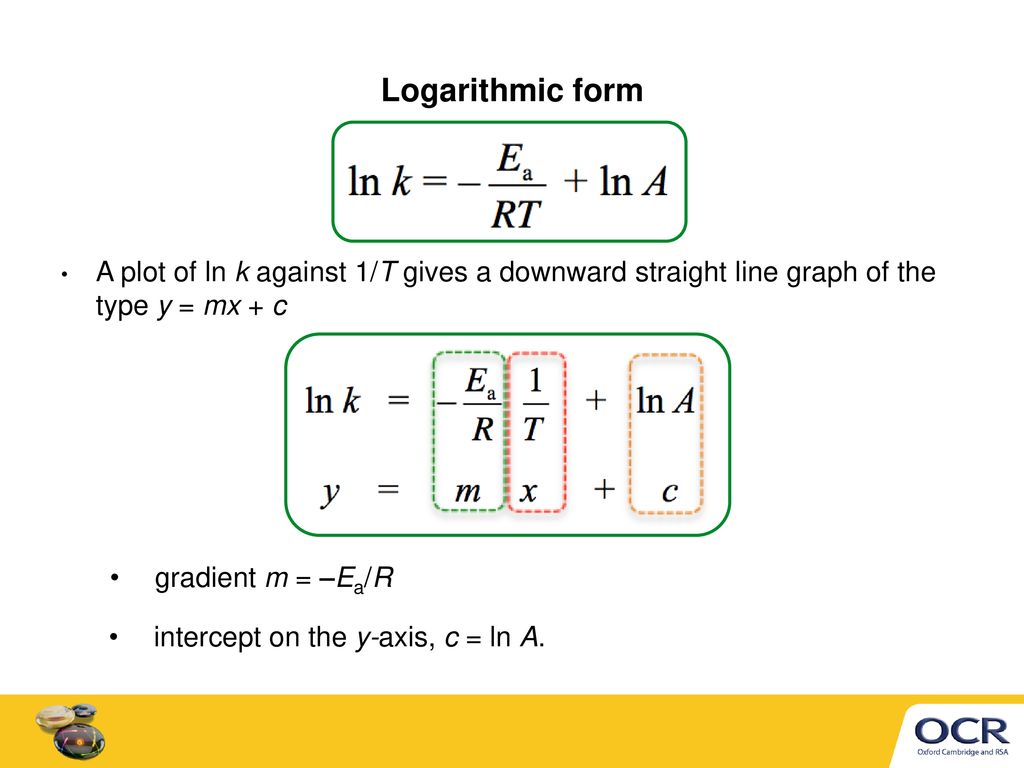
Arrhenius equation ymx+c. P and Z are not temperature dependent so the Arrhenius Equation is usually used as k = A e (Ea/RT) So in this case A (the preexponential factor) takes the place of both You should know the Maths expression for a straight line Y = mX c Where m = _____ & c = _____ If we plotted a graph of ln k vs 1/T QUESTION Use the Graph below to. E a is the activation energy of the reaction, which Arrhenius considered as the minimum energy that a molecule must have to posses to react Taking logarithm on both side of the equation (1) The above equation is of the form of a straight line y = mx c A plot of ln k Vs 1/T gives a straight line with a negative slope – E a /R If the rate. Arrhenius equation by the relationship ln is a logarithmic term ( loge NOT log10 ) (it can be obtained from a calculator) Graphical method • convert and rearrange the equation into the straight line formula y = mx c ln k = (Ea ⁄ R) 1⁄T ln A y = m x c • y axis plot ln k • x axis plot 1⁄T.
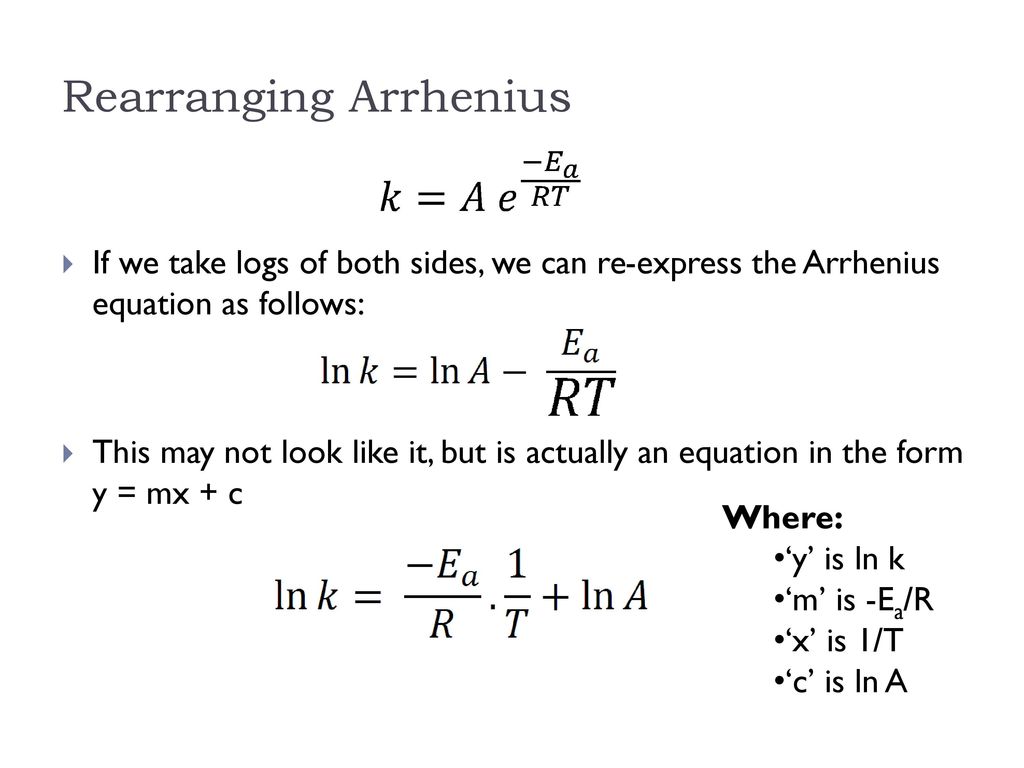
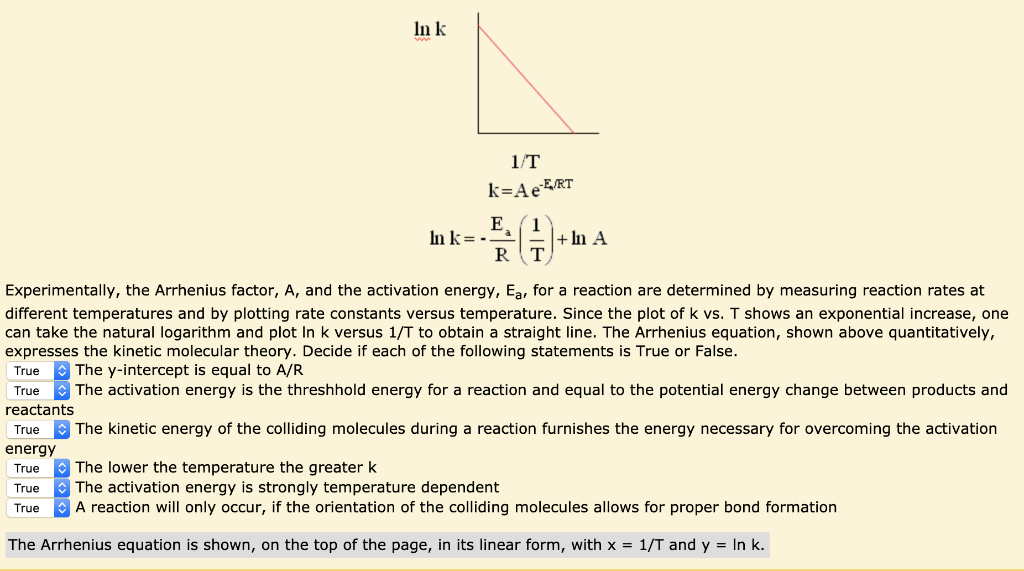
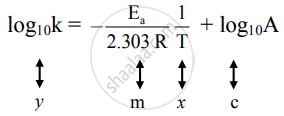
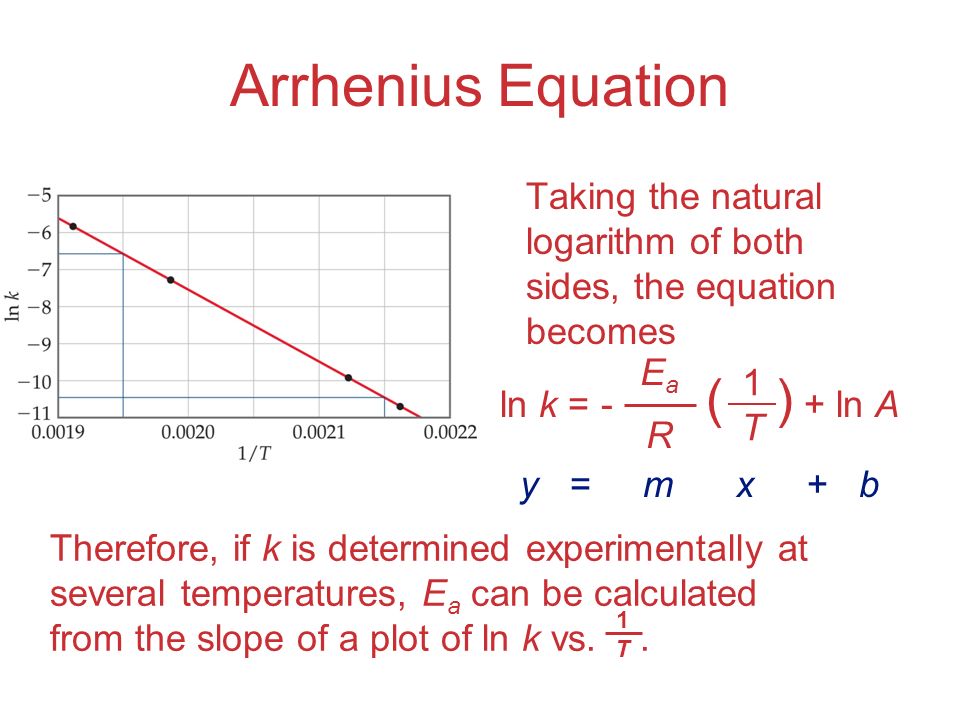
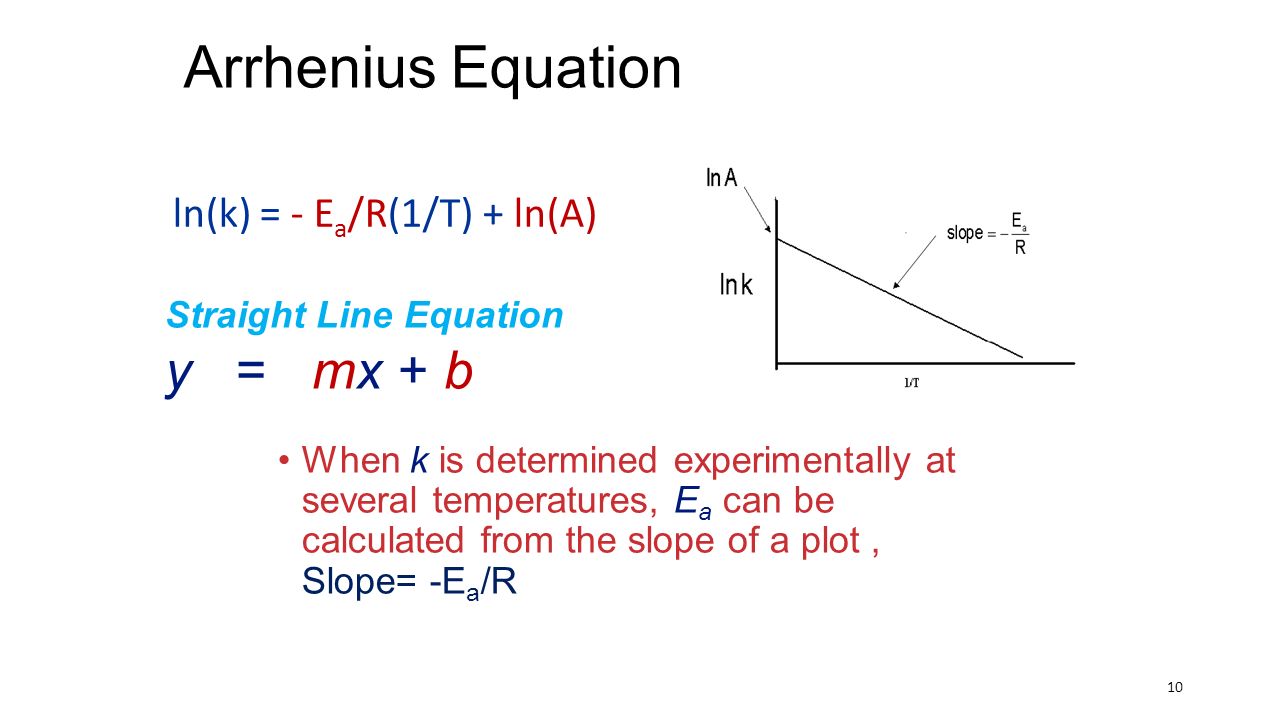
"Linearized" Arrhenius Equation The Arrhenius equation (Equation \ref{eq1}) can be rearranged to deal with specific situations For example, taking the logarithm of both sides yields the equation above in the form y=mxb. A broad generalization of the Arrhenius equation is to say the reaction rate for many chemical reactions doubles for every increase in 10 degrees Celsius or Kelvin While this "rule of thumb" isn't always accurate, keeping it in mind is a good way to check whether a calculation made using the Arrhenius equation is reasonable. To plot the graph, you use the log form of the Arrhenius equation log k = Ea/RT ln A This is in the form of a graph, y = mx c If you compare the two equations, you can see that y = log k m = Ea/R x = 1/T c = ln A So when you're plotting a graph of y against x, you plot log k against 1/T.
Accelerated Test Temperature are typically between 50 to 60C, most common 55°C Ambient storage temperature is typically between 22°C to 25°C 22°C will results in the shortest test duration Conservative / common Q10 is 2 for medical devices Relative Humidity (RH) is not a factor in the Arrhenius equation. This plot is called an "Arrhenius plot" Problem MK11 Recall that y = mx b a) In a socalled “Arrhenius plot” plot, what is the slope?. The _ of the Arrhenius equation represents the proportion of molecules that exceed Ea and have sufficient energy for a reaction to take place a plot of _ against 1/T gives a straight line graph of the type y = mx c 1 / T a plot of ln k against _ gives a straight line graph of the type y = mx c Ea / R.
The Arrhenius equation can be rewritten as Again, an equation of the form y = mx b is generated, indicating that a semilog plot of K app vs the reciprocal of the absolute temperature (1/T) should yield a straight line with a negative slope equal to E a /R This line can be extrapolated to the value of 1/T that corresponds to room. The Arrhenius equation is #k = Ae^(E_a//RT)# where #k# is the rate constant #A# is the preexponential factor;. With multiple variables and only one equation we won’t be able to fully solve the equation to a numeric answer, we can only rearrange it better to leave x by itself to satisfy this question First subtract the c from both sides of the equation to.
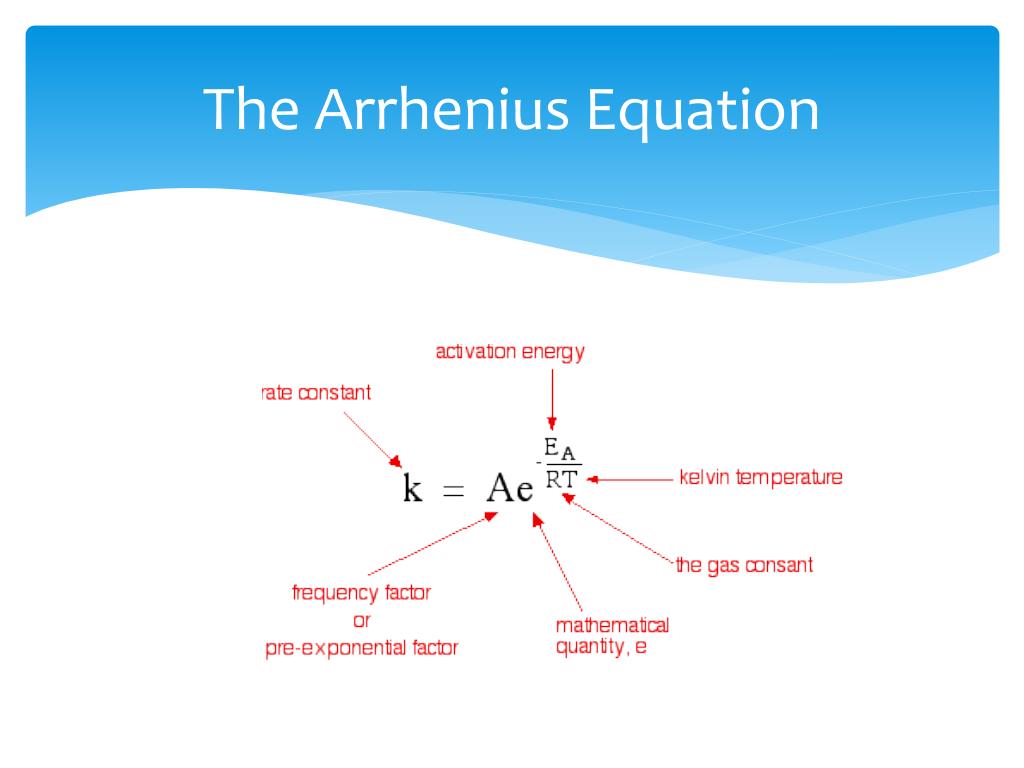
We can use the Arrhenius equation to relate the activation energy and the rate constant, k, of a given reaction \(k=A{e}^{\text{−}{E}_{\text{a}}\text{/}RT}\) In this equation, R is the ideal gas constant, which has a value 14 J/mol/K, T is temperature on the Kelvin scale, E a is the activation energy in joules per mole, e is the constant 271, and A is a constant called the frequency. Arrhenius equation by the relationship ln is a logarithmic term ( loge NOT log10 ) (it can be obtained from a calculator) Graphical method • convert and rearrange the equation into the straight line formula y = mx c ln k = (Ea ⁄ R) 1⁄T ln A y = m x c • y axis plot ln k • x axis plot 1⁄T. This equation is of the form of straightline y = mx c The Arrhenius plot of log 10 k versus `1/"T"` gives a straight line as shown in the diagram A slope of the line is `"E"_"a"/(2303 "R")` with its intercept being log 10 A.
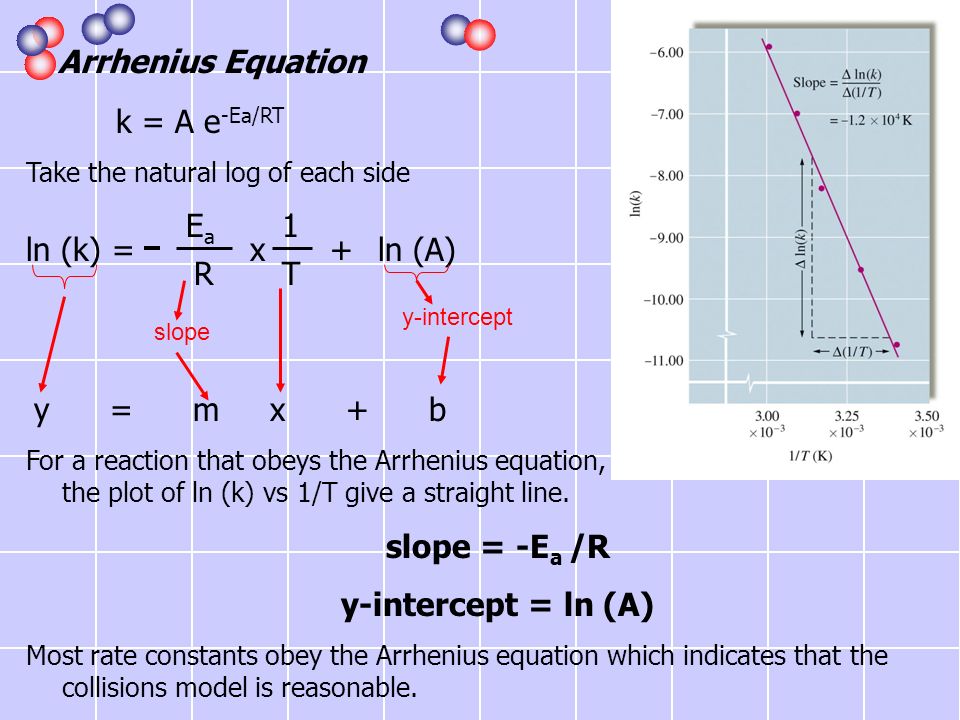
The Arrhenius equation What the various symbols mean Starting with the easy ones Temperature, T To fit into the equation, this has to be meaured in kelvin The gas constant, R This is a constant which comes from an equation, pV=nRT, which relates the pressure, volume and temperature of a particular number of moles of gas It turns up. E a is the activation energy of the reaction, which Arrhenius considered as the minimum energy that a molecule must have to posses to react Taking logarithm on both side of the equation (1) The above equation is of the form of a straight line y = mx c A plot of ln k Vs 1/T gives a straight line with a negative slope – E a /R If the rate. The integrated form of the Arrhenius equation is ln k = Ea/RT ln A which corresponds to y = mx b As you can see it corresponds to the slope intercept equation for a straight line where y = ln k, m (slope) = Ea/R, x = 1/T, and b = ln A Plot ln k in the y axis and 1/T on the x axis and then use Excel's "trendline" to get the slope.
Using the Arrhenius equation In reality, the basic form of the Arrhenius equation is not very convenient for graphing or analysing date To analyse experiments at different temperatures we usually use the natural log form of the equation k = AeEa/RT taking natural logs throughout this gives lnk = lnA Ea/RT. #E_a# is the activation energy #R# is the universal. A subtle rearrangement of this formula gives an equation that looks linear Notice the correspondence between terms of the Arrhenius expression and y = mx b This means if we measure a set of rates (k) and temperatures, T, we can create a plot of ln(k) vs 1/T.
Using the Arrhenius equation In reality, the basic form of the Arrhenius equation is not very convenient for graphing or analysing date To analyse experiments at different temperatures we usually use the natural log form of the equation k = AeEa/RT taking natural logs throughout this gives lnk = lnA Ea/RT. The Arrhenius equation can be rewritten as Again, an equation of the form y = mx b is generated, indicating that a semilog plot of K app vs the reciprocal of the absolute temperature (1/T) should yield a straight line with a negative slope equal to E a /R This line can be extrapolated to the value of 1/T that corresponds to room. The Arrhenius Equation As Used in Medical Device Accelerated Aging –Summary (ASTM F1980) 29 63 assumes that the chemical reactions involved in the deterioration of materials follow the Arrhenius reaction rate function A 10°C increase in temperature of a homogeneous process results in, approximately, a two times change in the rate.
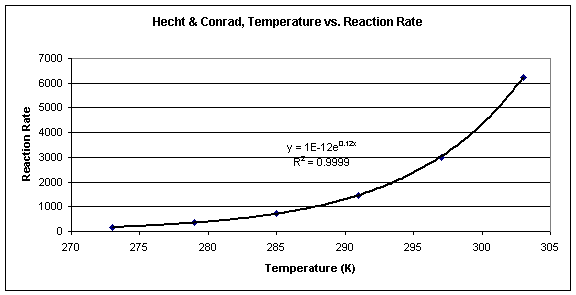
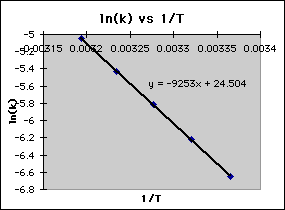
Arrhenius Equation (y = mx b) By using the straightline or nonexponential form of the Arrhenius equation we can determine either the frequency factor or the activation energy of a chemical reaction PRACTICE The rate constant for a reaction was measured as a function of temperature Based on the plot of ln k versus 1/T, what is the. Problem MK12 Using the following data, construct an Arrhenius plot and determine the activation energy (in both kcal/mol and kJ/mol) and the preexponential factor. The Arrhenius equation is a simple but remarkably accurate formula for the temperature dependence of the reaction rate constant, and therefore, the rate of a chemical reaction The equation was first proposed by Svante Arrhenius in 14 Five years later, in 18, Dutch chemist J H van ‘t Hoff provided physical justification and.
To plot the graph, you use the log form of the Arrhenius equation log k = Ea/RT ln A This is in the form of a graph, y = mx c If you compare the two equations, you can see that y = log k m = Ea/R x = 1/T c = ln A So when you're plotting a graph of y against x, you plot log k against 1/T. Y is ln(k), x is 1/T, and m is E a /R The activation energy for the reaction can be determined by finding the slope of the line. This plot is called an "Arrhenius plot" Problem MK11 Recall that y = mx b a) In a socalled “Arrhenius plot” plot, what is the slope?.
Arrheniusmcd SE Van Bramer 7/3/01 Chemistry 146 Lecture Problems Arrhenius Equation The Arrhenius Equation k A e E a RT From Example 218 in Kask and Rawn A 1010 14sec 1 E a 75 10. How to use the Arrhenius equation to calculate the activation energy And this is in the form of y=mxb, right?. So if you graph the natural log of the rate constant on the y axis and one over the temperature on the x axis, you're going to get a straight line And the slope of that straight line m is equal to Ea over R.
#E_a# is the activation energy #R# is the universal. Texy = mx c /tex where y = ln(k), c = ln(A), x = 1/T, m = Ea/R I derived nearly this very same formula for you from the Arrhenius Equation, in my previous post Go over it again, and in the place of k, simply substitute Ro/Ao, where Ro is the initial rate (the term on the LHS of the above rate equation). Being in the form of a straight line equation \(y = c m \times x\) Again for the sake of these question you do not necessarily have to understand WHY this equation matches the equation of a straight line, only that the gradient and y intercept help you find the above unknowns.
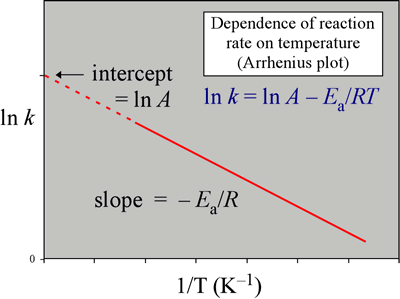
#E_a# is the activation energy #R# is the universal. Definition On a pressure–temperature (P–T) diagram, the line separating the two phases is known as the coexistence curve The Clausius–Clapeyron relation gives the slope of the tangents to this curve Mathematically, = =, where / is the slope of the tangent to the coexistence curve at any point, is the specific latent heat, is the temperature, is the specific volume change of the phase. A closer look at the Arrhenius equation reveals that the natural logarithm form of the Arrhenius equation is in the form of \(y = mx b\) In other words, it is similar to the equation of a straight line \ \ln k=\ln A \dfrac{E_{a}}{k_{B}T} \ where temperature is the independent variable and the rate constant is the dependent variable.
This is the Arrhenius Equation used in this experiment, which is in y = mxb form k stands for chemical reaction rate, A is pre=exponential factor, Eais activation energy, R is gas constant, which is 14 J K1 mol1, and T is temperature in Kelvin Therefore, this hypothesis is justified because the synthesis of coordination compounds will. Ln k = ln (A) ( − E a R T) = l n ( A) − ( E a R T) = l n ( A) − ( E a R) ( 1 T) The equation here is a straight line, y = mx c because ln (A) is constant and has a slope denoted by m = EaR When we plot the log of the rate constant, ln K on the Yaxis and the absolute temperature inverse (1T) on the Xaxis, the graph that results is referred to as the Arrhenius plot. Arrhenius Equation (y = mx b) By using the straightline or nonexponential form of the Arrhenius equation we can determine either the frequency factor or the activation energy of a chemical reaction PRACTICE The rate constant for a reaction was measured as a function of temperature Based on the plot of ln k versus 1/T, what is the.
Arrhenius equation by the relationship ln is a logarithmic term ( loge NOT log10 ) (it can be obtained from a calculator) Graphical method • convert and rearrange the equation into the straight line formula y = mx c ln k = (Ea ⁄ R) 1⁄T ln A y = m x c • y axis plot ln k • x axis plot 1⁄T. The Arrhenius equation is #k = Ae^(E_a//RT)# where #k# is the rate constant #A# is the preexponential factor;. Taking the natural logarithm of Arrhenius equation yields = − Rearranging yields = − This has the same form as an equation for a straight line = , where x is the reciprocal of T So, when a reaction has a rate constant that obeys Arrhenius equation, a plot of ln k versus T −1 gives a straight line, whose gradient and intercept can be used to determine E a and A.
The Arrhenius equation can be rewritten as Again, an equation of the form y = mx b is generated, indicating that a semilog plot of K app vs the reciprocal of the absolute temperature (1/T) should yield a straight line with a negative slope equal to E a /R This line can be extrapolated to the value of 1/T that corresponds to room. The Arrhenius equation is #k = Ae^(E_a//RT)# where #k# is the rate constant #A# is the preexponential factor;. The Arrhenius equation is a simple but remarkably accurate formula for the temperature dependence of the reaction rate constant, and therefore, the rate of a chemical reaction The equation was first proposed by Svante Arrhenius in 14 Note that this equation is of the form latexy=mxb/latex, and creating a plot of ln(k).
We can use the Arrhenius equation to relate the activation energy and the rate constant, k, of a given reaction \(k=A{e}^{\text{−}{E}_{\text{a}}\text{/}RT}\) In this equation, R is the ideal gas constant, which has a value 14 J/mol/K, T is temperature on the Kelvin scale, E a is the activation energy in joules per mole, e is the constant 271, and A is a constant called the frequency. Now compare the above equation with the equation of straight line we get y=mxc (4) Hence by plotting the graph between 1/T on xaxis and lnk on yaxis then antilog of intercept gives the value of rate constant and the slope of straightline will give the value of –Ea/R. B) What is the intercept?.
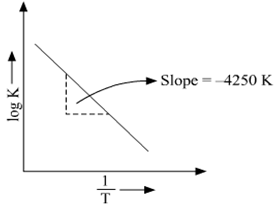
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators. This is a plot of a linear form of the Arrhenius equation In this form of the equation, the slope of this line is equal to Ea/R So,128X10^4 K = Ea / 14 J/molK Ea = 106X10^5 J/mol = 106 kJ/mol. Notice that when the Arrhenius equation is rearranged as above it is a linear equation with the form y = mx b;.
Now compare the above equation with the equation of straight line we get y=mxc (4) Hence by plotting the graph between 1/T on xaxis and lnk on yaxis then antilog of intercept gives the value of rate constant and the slope of straightline will give the value of –Ea/R. Voiceover We've already seen one form of the Arrhenius equation Which says that the rate constant k is equal to the frequency factor A times e to the negative ea over RT where ea is the activation energy, R is the gas constant, and T is the temperature. Since ln (A) is a constant, the equation corresponds to that of a straight line (y = mx c) whose slope (m) is E a /R When the logarithm of the rate constant (ln K) is plotted on the Yaxis and the inverse of the absolute temperature (1/T) is plotted on the Xaxis, the resulting graph is called an Arrhenius plot.
Y Mx C The Student Room
Arrhenius Equation Wikipedia

Arrhenius Equation Wikiwand

Arrhenius Equation Wikiwand
How Can You Graphically Find The Activation Energy Of The Reaction From The Above Expression Sarthaks Econnect Largest Online Education Community
Arrhenius Equation Which Graph Is Right The Math Doctors

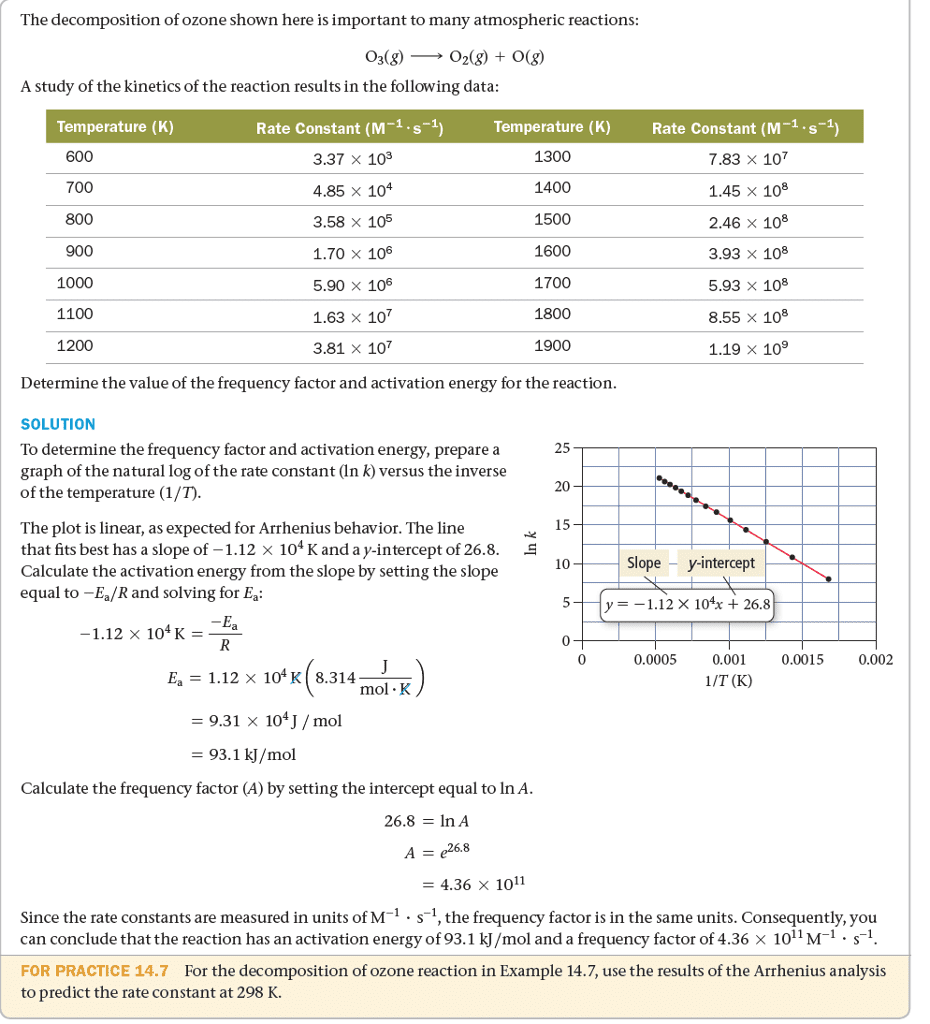
Chapter13 Chemical Kinetics
Pmt Physicsandmathstutor Com Download Chemistry A Level Notes Ocr A 5 Physical Chemistry Transition Elements Calculations for energetics Pdf
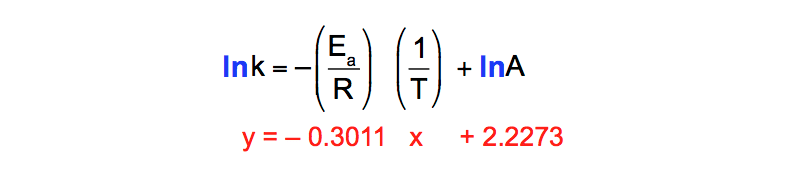
Arrhenius Equation Chemistry Video Clutch Prep
Ap Chemistry Chapter 14 Outline

Ib Chemistry Ellesmere College 16 2 Activation Energy

The Pharmaceutics And Compounding Laboratory

A Student Runs An Experiment In The Lab And Then Uses The Data To Prepare An Arrhenius Plot Of The Brainly Com
11 0 Reaction Kinetics Objectives 1 Define Reaction Rate Average Rate Instantaneous Rate And Initial Rate 2 Determine The Reaction Ppt Download
Arrhenius Kinetics Analysis
Rate Constant K Of A Reaction Varies With Temperature T According To The Equation Where Ea Is The Activation Energy When A Graph Is Plotted For A Straight Line With A Slope
4 8 Temperature And Rate Chemistry Libretexts

Arrhenius Equation Constants And Thermodynamic Analysis Of Ch And H
Determining Activation Energy

What Is The Arrhenius Equation Written In Y Mx B Format Lnae Which Part Of Course Hero

Plotting Data For A First Order Reaction Video Khan Academy
6 2 3 3 The Arrhenius Law Activation Energies Chemistry Libretexts
Recap 1 Two Species P And Q React Together According To The Following Equation P Q R The Accepted Mechanism For This Reaction Is P P P2 Fast Ppt Download
Oneclass I Do Not Get This So Please Explain When Answering Explain How Did They Figure Out The Slo

Kin Arrhenius Equation Notes Studocu

Hewxln9phe3w5m
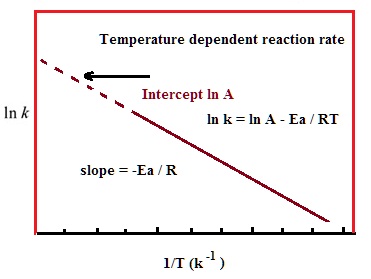
Table 1 The Main Differences Between Molecularity And Order Of Reaction

6 Plotting Of The Linear Form Of The Arrhenius Equation Provides A Download Scientific Diagram

Cbse Ncert Notes Class 12 Chemistry Chemical Kinetics
Http Barbara Cm Utexas Edu Courses Ch302s09 Files Lns09 Pdf

Chemistry A 3 1 9 2 Determination Of Rate Equation A Level Only Flashcards Quizlet
Ppt Rate Expression Powerpoint Presentation Free Download Id

Aqa A Level Chemistry Year 2 Paper 2 By Collins Issuu

Arrhenius Equation Expression Explanation Graph Solved Exercises
Solved Experimentally The Arrhenius Factor A And The A Chegg Com
Collision Model Energy Diagrams Arrhenius Equation Section 7 Chemical Kinetics Chapter Ppt Download
Q Tbn And9gcqkfaof231p1dk1n0sydwnfkifqyaar8du9nig Mc8p345spghh Usqp Cau

Question Video Estimating The Activation Energy Of A Reaction From Temperature And Reaction Rate

Ib Chemistry The Arrhenius Equation And Y Mx C Chem In 3 Episode 40 Youtube
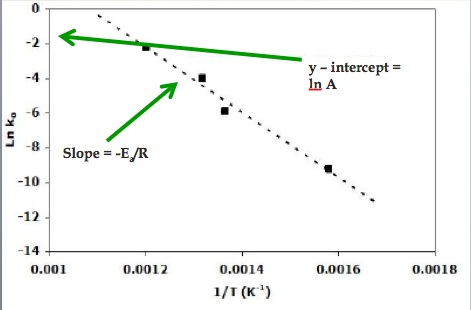
Track And Field Kinetics Ap Chemistry Olympics

Arrhenius Equation Activation Energy Derivation Clause Welllast
Plotting Linear Graphs In The Form Y Mx C Youtube
Q Tbn And9gcqh3rnlfhwnvgcq3r Pjnflttjeg2s3rcu5abtw5vcpihkj9gsx Usqp Cau

Chemical Dynamics Lecture 2 Chemical Kinetics Part 2

Activation Energy And The Arrhenius Equation Introductory Chemistry 1st Canadian Edition
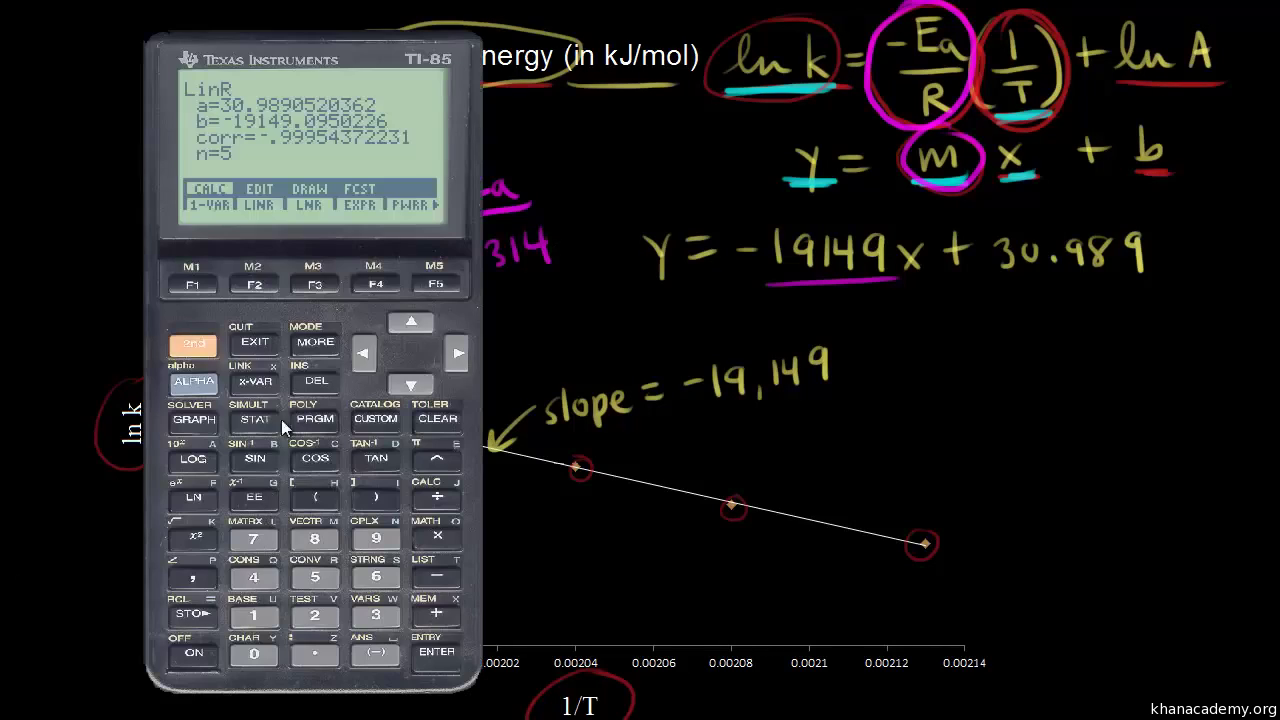
Using The Arrhenius Equation Video Khan Academy

Arrhenius Equation By Rachel Johnson

Nyb F09 Unit 2 Slides 26 57

A Level Chemistry Resources On Instagram Thermodynamics Entropy And The Arrhenius Equation Are 2 Of The Topics Chemistry Education Thermodynamics Chemistry

Hallarrhenius
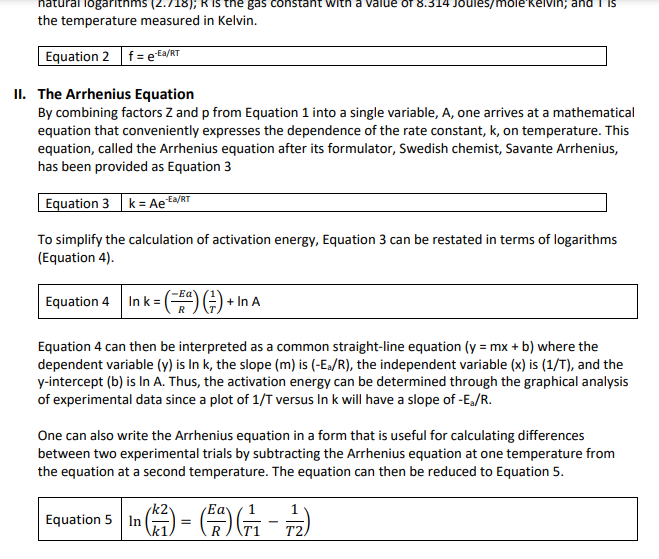
Solved Hi I Don T Know How To Solve X And Y From The Sec Chegg Com

Ib Chemistry The Arrhenius Equation And Y Mx C Chem In 3 Episode 40 Youtube

16 2 Arrhenius Equation To Find Ea Kerem S Chemistry Notes Ib

Arrhenius Equation Definition Examples And Theory Chemistry Dictionary
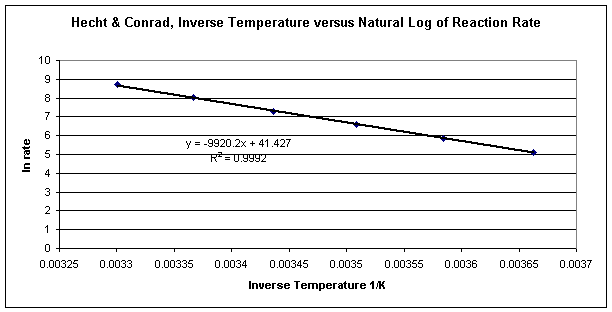
Arrhenius Kinetics Analysis
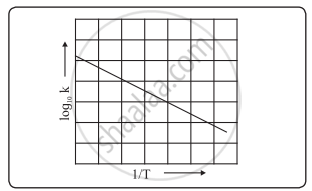
Answer The Following In Brief How Will You Determine Activation Energy Graphically Using The Arrhenius Equation Chemistry Shaalaa Com

Arrhenius Equation The Effect Of Temperature On Reaction Rate

Solved Section 1 Reactor Design In Chemical Engineering Chegg Com

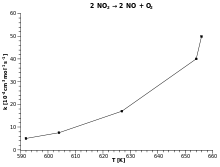
The Temperature Dependence Of Rate Constant K Of A Chemic
6 Plotting Of The Linear Form Of The Arrhenius Equation Provides A Download Scientific Diagram
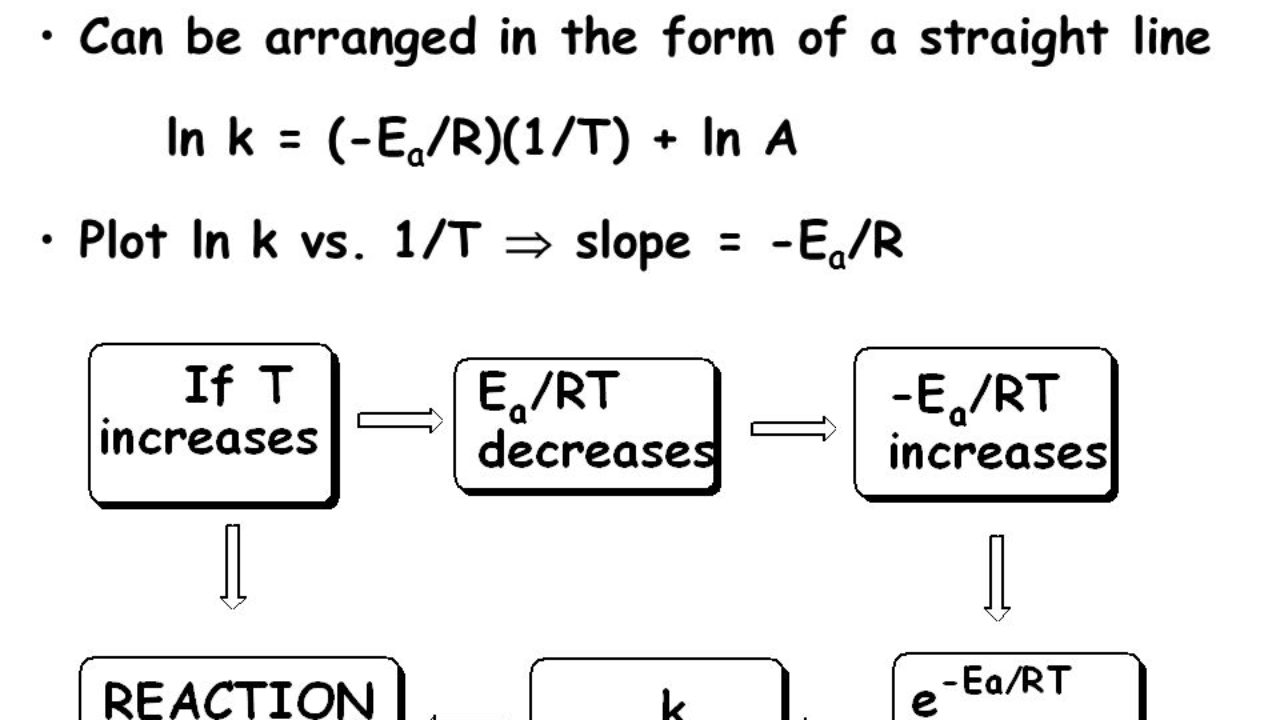
Arrhenius Equation Log K Ea 2 303 Rt Log A

Solved Q5 Write Down The Arrhenius Equation And Compare Chegg Com

Kinetics Arrhenius Equation Calculating Activation Energy From Rate Data Advanced A Level Gce Revision Notes

Arrhenius Equation Chemistry Video Clutch Prep
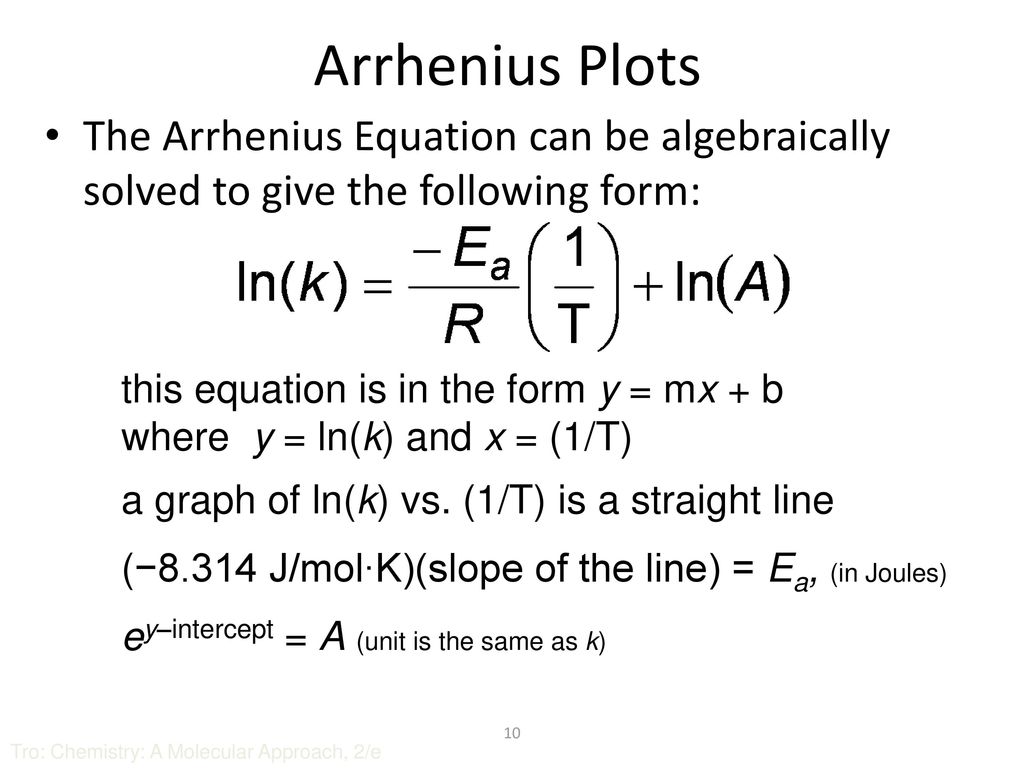
Chapter 13 Chemical Kinetics Ppt Download

Arrhenius Equation Wikiwand
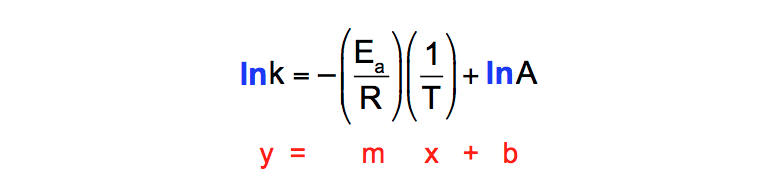
Arrhenius Equation Chemistry Video Clutch Prep

Arrhenius Equation Geochemistry I Lecture Slides Docsity

Effect Of Temperature On The Rate Constant Arrhenius Equation Pptx Activation Energy Reaction Rate

Suppose That Data Relating The Rate Constant And Temperature Results In A Line The Slope For Homeworklib

Arrhenius Equation Arrhenius Equation Relationship Between Rate And T K A U00d7 E Ea Rt Temperature In K Gas Constant 8 314 J Mol K Activation Energy Course Hero

In The Arrhenius Equation For A Certain Reaction The Values Of A And Ea Are 4 10 13 S 1 And 98 6 Kj Mol 1 Respectively If The Reaction Is Of First Order

Oneclass If You Were Going To Graphically Determine The Activation Energy Of This Reaction What Poi

Question Video Activation Energy From Arrhenius Plot Nagwa

Explain Linear Or Straight Line Graphs Qs Study

Arrhenius Equation Expression Explanation Graph Solved Exercises
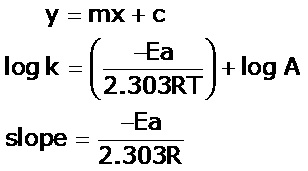
Cbse Class 12 Chemistry Notes Chemical Kinetics Arrhenius Equations Aglasem Schools

Section 1 Reactor Design In Chemical Engineering Chegg Com
Answer The Following In Brief How Will You Determine Activation Energy Graphically Using The Arrhenius Equation Chemistry Shaalaa Com
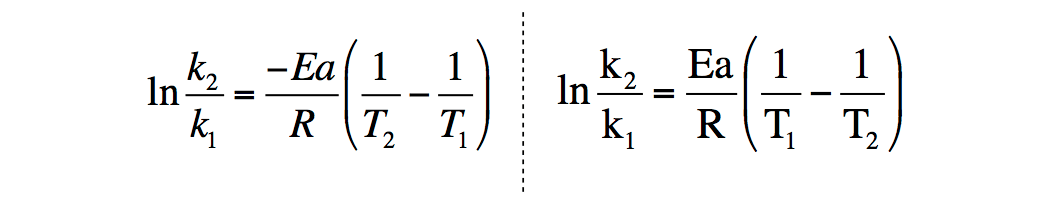
Arrhenius Equation Chemistry Video Clutch Prep
Solved This Is The Arrhenius Equation Ea K Ae Rt This Is The Linearized Arrhenius Equation In K Ea R In A Given The Meaning Of Linea Course Hero
The Arrhenius Equation Ppt Download
Chapter 12 Chemical Kinetics How Often Does Kinetics Appear On The Exam Multiple Choice 4 8 2 5 Questions Free Response Almost Every Year Kinetics Ppt Download

Arrhenius Equation Derivation Youtube
Arrhenius Equation Activation Enthalpy Classifieds App32 S Diary

Forms Of The Arrhenius Equation Video Khan Academy

Arrhenius Equation
Arrhenius Equation Calculator
Q Tbn And9gcqh3rnlfhwnvgcq3r Pjnflttjeg2s3rcu5abtw5vcpihkj9gsx Usqp Cau
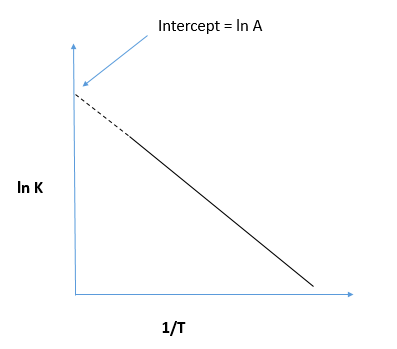
In Arrhenius Plot Intercept Is Equal To A Dfracear Class 11 Chemistry Cbse
Q Tbn And9gcr7pw1tq5iw9zwrmybnm3f Cihaa8n15q4gudmjeiagvyzbxq5c Usqp Cau

Arrhenius Equation

Chemical Kinetics By Prof Sanaa Taher Arab Physical Chemistry Ppt Download

Arrhenius Equation Chemistry Video Clutch Prep

Using Excel For Linear Regression

Arrhenius Plot An Overview Sciencedirect Topics
Activation Energy E A Is The Minimum Energy That Reactants Must Have To Form Products The Height Of The Potential Barrier Sometimes Called The Energy Ppt Download

Arrhenius Equation Expression Explanation Graph Solved Exercises



